Abstract
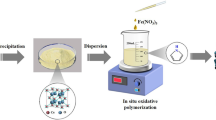
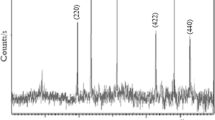
Aim of this work is to synthesizing a new highly efficient adsorbent as of magnesium oxide (MgO2) entrapped Polypyrrole (Ppy) nanocomposite preparation for toxic pollutant of fluoride removal form drinking water. The synthesized MgO2/Ppy hybrid nanocomposite shows an extraordinary defluoridation capacity of 4328 mg F− Kg−1 in the room temperature through batch adsorption technique. It was performed to know the effect of various parameters such as contact time, initial concentration, pH, competitor ions and temperature. The structural and morphological changes on the fluoride adsorbent in before and after were analyzed by using XRD, FTIR & SEM techniques. The adsorption isotherm of Freundlich, Langmuir, & Dubnin - Radushkevich isotherms were studied and the fluoride adsorption is well fixed with Langmuir isotherm model. The kinetic model studies were carried out by both diffusion and reaction based models. The mechanism of fluoride on the MgO2/Ppy nanocomposite is mainly influence the interaction of electrostatic adsorption and ion-exchange. The reusability and regeneration studies were performed for the reusability of the nanocomposite.








Similar content being viewed by others
References
S. Oguz, J. Hazard, Mater. 117, 227–233 (2005)
Y. Wang, E.J. Reardon, Activation and regeneration of a soil sorbent for defluoridation of drinking water. Appl. Geochem. 16, 531–539 (2001)
WHO, Guidelines for drinking-water quality first addendum to third edition, Volume 1 recommendations, (2006)
M.S. Onyango, Y. Kojima, O. Aoyi, E.C. Bernardo, H. Matsuda, Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci. 279, 341–350 (2004)
G. Singh, J. Majumdar, Water Environ. Res. 71, 36–42 (1998)
S.K. Adhikary, U.K. Tipnis, W.P. Harkare, K.P. Govindan, Defluoridation during desalination of brackish water by electrodialysis. Desalination 71, 301–312 (1989)
R. Simons, Trace element removal from ash dam waters by nanofiltration and diffusion dialysis. Desalination 89, 325–341 (1993)
M. Rajan, G. Alagumuthu, Study of fluoride affinity by zirconium impregnated walnut shell carbon in aqueous phase: kinetic and isotherm evaluation. J. Chem. 2013, Article ID 235048, 8 pages (2013)
I. Ali, V.K. Gupta, Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2007)
J. Chen, J. Feng, W. Yan, Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for Methylene Blue. J. Colloid Interface Sci. 475, 26–35 (2016)
M.L. Zhang, H.Y. Zhang, D. Xu, L. Han, D.X. Niu, B.H. Tian, J.A. Zhang, L.Y. Zhang, W.S. Wu, Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method. Desalination 271, 111–121 (2011)
M. Karthikeyan, K.K. Satheeshkumar, K.P. Elango, Removal of fluoride ions from aqueous solution by conducting polypyrrole. J. Hazard. Mater. 167, 300–305 (2009)
A.C. Zettlemoyer, E.A. Zettlemoyer, W.C. Walker, Active Magnesia. II. Adsorption of Fluoride from Aqueous Solution. J. Am. Chem. Soc. 69, 1312–1315 (1947)
P. Venkateswarlu, D.N. Rao, Indian J. Med. Res. 41, 473–477 (1953)
S.M. Rao, P. Mamatha, Curr. Sci. 87, 942–947 (2004)
M.V.L. Ramon, F. Stoeckli, C.M. Castilla, F.C. Marin, On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 37, 1215–1221 (1999)
A.A. Khan, R.P. Singh, Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids Surf. 24, 33–42 (1987)
A. Nagaraj, K.K. Sadasivuni, M. Rajan, Investigation of lanthanum impregnated cellulose, derived from biomass, as an adsorbent for the removal of fluoride from drinking water. Carbohydr. Polym. 176, 402–410 (2017)
L. Liu, Z. Cui, Q. Ma, W. Cui, X. Zhang, One-step synthesis of magnetic iron–aluminum oxide/graphene oxide nanoparticles as a selective adsorbent for fluoride removal from aqueous solution. RSC Adv. 6, 10783–10791 (2016)
K. Pandi, In SituFabrication of Magnetic Iron Oxide over Nano-hydroxyapatite Gelatin Eco-polymeric Composite for Defluoridation Studies. N. Viswanathan J Chem Eng Data. 61, 571–578 (2016)
G. Alagumuthu, M. Rajan, Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem. Eng. J. 158, 451–457 (2010)
S. Gao, R. Sun, Z. Wei, H. Zhao, H. Li, F. Hu, Size-dependent defluoridation properties of synthetic hydroxyapatite. J. Fluor. Chem. 130, 550–556 (2009)
N. Chen, Z. Zhang, C. Feng, N. Sugiura, M. Li, R. Chen, Fluoride removal from water by granular ceramic adsorption. J. Colloid Interface Sci. 348, 579–584 (2010)
Z. Li, S. Deng, X. Zhang, W. Zhou, J. Huang, G. Yu, Removal of fluoride from water using titanium-based adsorbents. Front. Environ. Sci. Eng. China 4, 414–420 (2010)
M. Bhaumik, S. Agarwal, V.K. Gupta, A. Maity, Enhanced removal of Cr(VI) from aqueous solutions using polypyrrole wrapped oxidized MWCNTs nanocomposites adsorbent. J. Colloid Interface Sci. 470, 257–267 (2016)
H.M.F. Freundlich, Over the adsorption in solution. J. Phys. Chem. 57, 385–470 (1906)
I. Langmuir, The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 38, 2221–2295 (1916)
S. Karahan, M. Yurdakoc, Y. Seki, K. Yurdakoc, Removal of boron from aqueous solution by clays and modified clays. J. Colloid Interface Sci. 293, 36–42 (2006)
Saha, S. Chowdhury, Thermodynamics In Tech. 349–364 (2011)
V.J. Inglezakis, A.A. Zorpas, Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin. Water Treat. 39, 149–157 (2012)
M. Mohapatra, D. Hariprasad, L. Mohapatra, S. Anand, B.K. Mishra, Mg-doped nano ferrihydrite—A new adsorbent for fluoride removal from aqueous solutions. Appl. Surf. Sci. 258, 4228–4236 (2012)
Acknowledgements
M. Rajan is grateful to the UGC-University Grants Commission, India, for providing financial support under the schemes of “UGC-MRP Grants”(Ref: F. No. 43-187/2014 (SR)) and Department of Science and Technology, Science and Engineering Research Board (DST-SERB) (Ref: YSS/2015/001532; New Delhi, India) and also acknowledges the PURSE program for the purchase of SEM and FT-IR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagaraj, A., Govindaraj, D. & Rajan, M. Magnesium oxide entrapped Polypyrrole hybrid nanocomposite as an efficient selective scavenger for fluoride ion in drinking water. emergent mater. 1, 25–33 (2018). https://doi.org/10.1007/s42247-018-0001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-018-0001-5