Abstract
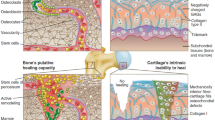
Osteochondral (OC) lesions are characterized by defects in two different zones, the cartilage region and subchondral bone region. These lesions are frequently associated with mechanical instability, as well as osteoarthritic degenerative changes in the knee. The lack of spontaneous healing and the drawbacks of the current treatments have increased the attention from the scientific community to this issue. Different tissue engineering approaches have been attempted using different polymers and different scaffolds’ processing. However, the current conventional techniques do not allow the full control over scaffold fabrication, and in this type of approaches, the tuning ability is the key to success in tissue regeneration. In this sense, the researchers have placed their efforts in the development of solid free-form (SFF) techniques. These techniques allow tuning different properties at the micro–macro scale, creating scaffolds with appropriate features for OC tissue engineering. In this review, it is discussed the current SFF techniques used in OC tissue engineering and presented their promising results and current challenges.


Reprinted with permission from Ref. [19]

Reprinted with permission from Ref. [33]

Reprinted with permission from Ref. [40]
Similar content being viewed by others
References
Nooeaid P, Salih V, Beier JP, Boccaccini AR (2012) Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med 16(10):2247–2270. https://doi.org/10.1111/j.1582-4934.2012.01571.x
Dhollander AA, Guevara Sanchez VR, Almqvist KF, Verdonk R, Verbruggen G, Verdonk PC (2012) The use of scaffolds in the treatment of osteochondral lesions in the knee: current concepts and future trends. J Knee Surg 25(3):179–186
Reichert JC, Wullschleger ME, Cipitria A, Lienau J, Cheng TK, Schutz MA, Duda GN, Noth U, Eulert J, Hutmacher DW (2011) Custom-made composite scaffolds for segmental defect repair in long bones. Int Orthop 35(8):1229–1236. https://doi.org/10.1007/s00264-010-1146-x
Yousefi AM, Hoque ME, Prasad RG, Uth N (2015) Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res Part A 103(7):2460–2481. https://doi.org/10.1002/jbm.a.35356
Oliveira JM, Rodrigues MT, Silva SS, Malafaya PB, Gomes ME, Viegas CA, Dias IR, Azevedo JT, Mano JF, Reis RL (2006) Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 27(36):6123–6137. https://doi.org/10.1016/j.biomaterials.2006.07.034
Levingstone TJ, Matsiko A, Dickson GR, O’Brien FJ, Gleeson JP (2014) A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater 10(5):1996–2004. https://doi.org/10.1016/j.actbio.2014.01.005
Zhou J, Xu C, Wu G, Cao X, Zhang L, Zhai Z, Zheng Z, Chen X, Wang Y (2011) In vitro generation of osteochondral differentiation of human marrow mesenchymal stem cells in novel collagen-hydroxyapatite layered scaffolds. Acta Biomater 7(11):3999–4006. https://doi.org/10.1016/j.actbio.2011.06.040
Huang X, Yang D, Yan W, Shi Z, Feng J, Gao Y, Weng W, Yan S (2007) Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials 28(20):3091–3100. https://doi.org/10.1016/j.biomaterials.2007.03.017
Jiang CC, Chiang H, Liao CJ, Lin YJ, Kuo TF, Shieh CS, Huang YY, Tuan RS (2007) Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res 25(10):1277–1290. https://doi.org/10.1002/jor.20442
Hutmacher DW, Sittinger M, Risbud MV (2004) Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 22(7):354–362. https://doi.org/10.1016/j.tibtech.2004.05.005
Sachlos E, Czernuszka JT (2003) Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cells Mater 5:29–39 (discussion 39–40)
Martin I, Miot S, Barbero A, Jakob M, Wendt D (2007) Osteochondral tissue engineering. J Biomech 40(4):750–765. https://doi.org/10.1016/j.jbiomech.2006.03.008
Houben A, Van Hoorick J, Van Erps J, Thienpont H, Van Vlierberghe S, Dubruel P (2017) Indirect rapid prototyping: opening up unprecedented opportunities in scaffold design and applications. Ann Biomed Eng 45(1):58–83. https://doi.org/10.1007/s10439-016-1610-x
Harris RA, Hague RJM, Dickens PM (2004) The structure of parts produced by stereolithography injection mould tools and the effect on part shrinkage. Int J Mach Tools Manuf 44(1):59–64. https://doi.org/10.1016/j.ijmachtools.2003.08.007
Skoog SA, Goering PL, Narayan RJ (2014) Stereolithography in tissue engineering. J Mater Sci Mater Med 25(3):845–856. https://doi.org/10.1007/s10856-013-5107-y
Weiguo B, Dichen L, Qin L, Xiang L, Weijie Z, Kunzheng W, Zhongmin J (2012) Fabrication of a bio-inspired beta-Tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyp J 18(1):68–80. https://doi.org/10.1108/13552541211193511
Bian W, Lian Q, Li D, Wang J, Zhang W, Jin Z, Qiu Y (2016) Morphological characteristics of cartilage-bone transitional structures in the human knee joint and CAD design of an osteochondral scaffold. BioMed Eng OnLine 15:82. https://doi.org/10.1186/s12938-016-0200-3
Zhang W, Lian Q (2014) Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: a one-year-period study in rabbit trochlea. BioMed Res Int 2014:746138. https://doi.org/10.1155/2014/746138
Castro NJ, O’Brien J, Zhang LG (2015) Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 7(33):14010–14022. https://doi.org/10.1039/c5nr03425f
Ronca A, Maiullari F, Milan M, Pace V, Gloria A, Rizzi R, De Santis R, Ambrosio L (2017) Surface functionalization of acrylic based photocrosslinkable resin for 3D printing applications. Bioact Mater 2(3):131–137. https://doi.org/10.1016/j.bioactmat.2017.04.002
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, Noh I, Mikos AG, Zhang S (2017) Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 137:37–48. https://doi.org/10.1016/j.biomaterials.2017.05.021
Fousova M, Vojtech D, Doubrava K, Daniel M, Lin CF (2018) Influence of inherent surface and internal defects on mechanical properties of additively manufactured Ti6Al4V alloy: comparison between selective laser melting and electron beam melting. Materials (Basel, Switzerland) 11(4):537. https://doi.org/10.3390/ma11040537
Zhang B, Pei X, Zhou C, Fan Y, Jiang Q, Ronca A, D’Amora U, Chen Y, Li H, Sun Y, Zhang X (2018) The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4 V scaffold for load-bearing bone reconstruction. Mater Des 152:30–39. https://doi.org/10.1016/j.matdes.2018.04.065
Endres M, Hutmacher DW, Salgado AJ, Kaps C, Ringe J, Reis RL, Sittinger M, Brandwood A, Schantz JT (2003) Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer-hydrogel matrices. Tissue Eng 9(4):689–702. https://doi.org/10.1089/107632703768247386
Heo SJ, Kim SE, Wei J, Hyun YT, Yun HS, Kim DH, Shin JW, Shin JW (2009) Fabrication and characterization of novel nano- and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J Biomed Mater Res Part A 89(1):108–116. https://doi.org/10.1002/jbm.a.31726
Heo SJ, Kim SE, Wei J, Kim DH, Hyun YT, Yun HS, Kim HK, Yoon TR, Kim SH, Park SA, Shin JW, Shin JW (2009) In vitro and animal study of novel nano-hydroxyapatite/poly(epsilon-caprolactone) composite scaffolds fabricated by layer manufacturing process. Tissue Eng Part A 15(5):977–989. https://doi.org/10.1089/ten.tea.2008.0190
Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW (2007) Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng 24(5):489–495. https://doi.org/10.1016/j.bioeng.2007.07.014
Ding C, Qiao Z, Jiang W, Li H, Wei J, Zhou G, Dai K (2013) Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 34(28):6706–6716. https://doi.org/10.1016/j.biomaterials.2013.05.038
Holmes B, Zhu W, Li J, Lee JD, Zhang LG (2015) Development of novel three-dimensional printed scaffolds for osteochondral regeneration. Tissue Eng Part A 21(1–2):403–415. https://doi.org/10.1089/ten.tea.2014.0138
De Santis R, Gloria A, Russo T, Ronca A, D’Amora U, Negri G, Ronca D, Ambrosio L (2016) Viscoelastic properties of rapid prototyped magnetic nanocomposite scaffolds for osteochondral tissue regeneration. Procedia CIRP 49:76–82. https://doi.org/10.1016/j.procir.2015.07.037
Woodfield TBF, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA (2004) Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 25(18):4149–4161. https://doi.org/10.1016/j.biomaterials.2003.10.056
Malda J, Woodfield TB, van der Vloodt F, Kooy FK, Martens DE, Tramper J, van Blitterswijk CA, Riesle J (2004) The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 25(26):5773–5780. https://doi.org/10.1016/j.biomaterials.2004.01.028
Barron V, Merghani K, Shaw G, Coleman CM, Hayes JS, Ansboro S, Manian A, O’Malley G, Connolly E, Nandakumar A, van Blitterswijk CA, Habibovic P, Moroni L, Shannon F, Murphy JM, Barry F (2015) Evaluation of cartilage repair by mesenchymal stem cells seeded on a PEOT/PBT scaffold in an osteochondral defect. Ann Biomed Eng 43(9):2069–2082. https://doi.org/10.1007/s10439-015-1246-2
Di Luca A, Szlazak K, Lorenzo-Moldero I, Ghebes CA, Lepedda A, Swieszkowski W, Van Blitterswijk C, Moroni L (2016) Influencing chondrogenic differentiation of human mesenchymal stromal cells in scaffolds displaying a structural gradient in pore size. Acta Biomater 36:210–219. https://doi.org/10.1016/j.actbio.2016.03.014
Criscenti G, Longoni A, Di Luca A, De Maria C, van Blitterswijk CA, Vozzi G, Moroni L (2016) Triphasic scaffolds for the regeneration of the bone-ligament interface. Biofabrication 8(1):015009. https://doi.org/10.1088/1758-5090/8/1/015009
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ (2012) Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 18(1):33–44. https://doi.org/10.1089/ten.TEC.2011.0060
Jin-Hyung S, Jung-Seob L, Jong Young K, Dong-Woo C (2012) Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng 22(8):085014
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18(11–12):1304–1312. https://doi.org/10.1089/ten.TEA.2011.0543
Cohen DL, Lipton JI, Bonassar LJ, Lipson H (2010) Additive manufacturing for in situ repair of osteochondral defects. Biofabrication 2(3):035004. https://doi.org/10.1088/1758-5082/2/3/035004
Li L, Yu F, Shi J, Shen S, Teng H, Yang J, Wang X, Jiang Q (2017) In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci Rep 7(1):9416. https://doi.org/10.1038/s41598-017-10060-3
Acknowledgements
The authors would like to thank H2020-MSCA-RISE program, as this work is part of developments carried out in BAMOS project, funded from the European Union’s Horizon 2020 research and innovation program under grant agreement Nº 734156. The Portuguese Foundation for Science and Technology (FCT) distinctions attributed to J. Silva-Correia (IF/00115/2015) and J. Miguel Oliveira (IF/01285/2015) under the Investigator FCT program are greatly acknowledged. FCT/MCTES is also acknowledged for the PhD scholarship attributed to J. B. Costa (PD/BD/113803/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Costa, J.B., Silva-Correia, J., Reis, R.L. et al. Current advances in solid free-form techniques for osteochondral tissue engineering. Bio-des. Manuf. 1, 171–181 (2018). https://doi.org/10.1007/s42242-018-0017-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-018-0017-y