Abstract
Background
Biallelic mutations in the TBX19 gene cause severe early-onset adrenal failure due to isolated ACTH deficiency (IAD). This rare disease is characterized by low plasma ACTH and cortisol levels, with normal secretion of other pituitary hormones. Herein, we report a patient with IAD due to a novel TBX19 gene mutation, who is also of tall stature.
Case report
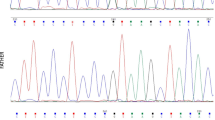
A 48/12-year-old girl was presented with loss of consciousness due to hypoglycemia. The patient was born at term with a birth weight of 3800 g. Her parents were first-degree cousins. She had a history of several hospitalizations for recurrent seizures, abdominal pain, and vomiting. At presentation, her weight and height were + 1.8 and + 2.2 SDS, respectively. Serum glucose was 25 mg/dl (1.4 mmol/L), with normal sodium, potassium, and insulin concentrations. The child was hypocortisolemic (0.1 μg/dl), and ACTH levels were extremely low (< 5.0 pg/ml). A diagnosis of IAD was made and hydrocortisone treatment was started. Hypoglycemic episodes, seizures, and recurrent gastrointestinal complaints disappeared after hydrocortisone replacement. Magnetic resonance imaging of the pituitary was normal. Whole exome sequencing revealed a novel homozygous c.302G > A (W101*) mutation in the TBX19 gene.
Conclusion
We report a new mutation in the TBX19 gene in a patient with isolated ACTH deficiency. While overgrowth is a known feature of some types of adrenal insufficiencies, including MC2R gene defects and POMC deficiency, it may be a novel feature for TPIT deficiency, as in our patient.


Similar content being viewed by others
References
Couture C, Saveanu A, Barlier A et al (2012) Phenotypic homogeneity and genotypic variability in a large series of congenital isolated ACTH-deficiency patients with TPIT gene mutations. J Clin Endocrinol Metab 97:486–495
Vallette- Kasic S, Brue T, Pulichino AM et al (2005) Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death explained by TPIT gene mutations. J Clin Endocrinol Metab 90:1323–1331
Pulichino AM, Vallette-Kasic S, Couture C, Brue T, Drouin J (2004) Tpit mutations reveal a new model of pituitary differentiation and account for isolated ACTH deficiency. Med Sci (Paris) 20:1009–1013
Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J (2003b) Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747
Lamolet B, Pulichino AM, Lamonerie T et al (2001) A pituitary cell-restricted T box factor Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859
Stenson PD, Mort M, Ball EV et al (2017) The human mutation database: towards a comphrehensive repository of inherited mutation data for medical research, genetic diagnosis and next generation sequencing studies. Hum Genet 136:665–677
Déchelotte P, Darcha C, Labbé A, Vanlieféringhen P, Beaufrère AM, Malpuech G (1994) Congenital adrenal hypoplasia due to isolated familial ACTH deficiency. Pediatr Pathol 14:377–380
Adzhubei I, Jordan DM, Sunyaev SR (2013) Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 7:Unit7.20
Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC (2012) SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 40:452–457
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11:361–362
Metherell LA, Savage MO, Dattani M et al (2004) TPIT mutations are associated with early onset, but not late-onset isolated ACTH deficieny. Eur J Endocrinol 151:463–465
Alsaleem M, Saadeh L, Misra A, Madani S 2016 Neonatal isolated ACTH deficiency (IAD): a potentialy life-threatening but treatable cause of neonatal cholestasis. BMC Case Rep. https://doi.org/10.1136/bcr-2016-215032
Unal E, Yıldırım R, Taş FF, Tekin S, Sen A, Haspolat YK (2018) A rare cause of neonatal hypoglycemia in two siblings: TBX19 gene mutation. Hormones (Athens) 17:269–273
Akcan N, Serakıncı N, Turkgenc B, Bundak R, Bahceciler N, Temel SG (2017) A novel TBX19 gene mutation in a case of congenital isolated adrenocorticotropic hormone deficiency presenting with recurrent respiratory tract infections. Front Endocrinol (Lausanne) 8:64
Pulichino AM, Vallette-Kasic S, Couture C et al (2003a) Human and mouse TPIT gene mutations cause early-onset pituitary ACTH deficiency. Genes Dev 17:711–716
Zachmann M, Girard J, Duc G, Illiq R, Prader A (1979) Low estriol during pregnancy caused by isolated ACTH- deficiency. Acta Paediatr Scand Suppl 277:26–31
Malpuech G, Vanlieferinghen P, Dechelotte P, Gaulme J, Labbe A, Guiot F (1988) Isolated familial adrenocorticotropin deficiency: prenatal diagnosis by maternal plasma estriol assay. Am J Med Genet 29:125–130
Weintrob N, Drouin J, Vallette- Kasic J et al (2006) Low estriol levels in the maternal triple-marker screen as a predictor of isolated adrenocorticotropic hormone deficiency caused by a new mutation in the TPIT gene. Pediatrics 117:322–327
Novoselova TV, Chan LF, Clark AJL (2018) Pathophysiology of melanocortin receptors and their accessory proteins. Best Pract Res Clin Endocrinol Metab 32:93–106
Fridmanis D, Roga A, Klovins J (2017) ACTH receptor (MC2R) specifity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne) 8:13
Naville D, Weber A, Genin E, Durand P, Clark AJ, Begeot M (1998) Exclusion of the adrenocorticotropin (ACTH) receptor (MC2R) locus in some families with ACTH resistance but no mutations of the MC2R coding sequence (familial glucocorticoid deficiency type 2). J Clin Endocrinol Metab 83:3592–3596
Yeh JK, Evans JF, Niu QT, Aloia JF (2006) A possible role for melanocortin peptides in longitudinal growth. J Endocrinol 191:677–686
Chida D, Nakagawa S, Nagai S et al (2007) Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A 104:18205–18210
Chung TT, Chan LF, Metherell LA, Clark AJ (2010) Phenotypis characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin Endocrinol 72:589–594
Anisimova AS, Rubtsov PM, Akulich KA, Dmitriev SE, Frolova E, Tiulpakov A (2017) Late diagnosis of POMC deficiency and in vitro evidence of residual translation from allele with c.-11C>a mutation. J Clin Endocrinol Metab 102:359–362
Darcan S, Can S, Goksen D, Asar G (2010) Transient salt wasting in POMC-deficiency due to infection induced stress. Exp Clin Endocrinol Diabetes 118:281–283
Özen S, Özcan N, Uçar SK, Gökşen D, Darcan Ş (2015) Unexpected clinical features in a female patient with proopiomelanocortin (POMC) deficiency. J Pediatr Endocrinol Metab 28:691–694
Aslan IR, Ranadive SA, Valle I, Kollipara S, Noble JA, Vaisse C (2014) The melanocortin system and insulin resistance in humans: insights from a patient with complete POMC deficiency and type 1 diabetes mellitus. Int J Obes 38:148–151
Ozsu E, Bahm A (2017) Delayed diagnosis of proopiomelanocortin (POMC) deficiency with type 1 diabetes in a 9-year-old girl and her infant sibling. J Pediatr Endocrinol Metab 30:1137–1140
Acknowledgements
The authors wish to express their gratitude to the parents and the patient who participated in this study.
Author contributions
Zehra Yavas Abali, Gozde Yesil, Tarik Kirkgoz, Sare Betul Kaygusuz, Mehmet Eltan, Serap Turan, Abdullah Bereket, and Tulay Guran conceptualized and designed the study. Zehra Yavas Abali and Tulay Guran drafted the initial manuscript. Serap Turan, Abdullah Bereket, and Tulay Guran critically reviewed the manuscript. Gozde Yesil and Tulay Guran performed the assays, analyzed data, and interpreted the results. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Patient confidentiality
The patient’s parents provided informed consent for publication of the submitted article and the results of the accompanying genetic analyses.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abali, Z.Y., Yesil, G., Kirkgoz, T. et al. Evaluation of growth and puberty in a child with a novel TBX19 gene mutation and review of the literature. Hormones 18, 229–236 (2019). https://doi.org/10.1007/s42000-019-00096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-019-00096-7