Abstract
Solid oxide electrolysis cells (SOECs) including the oxygen ion-conducting SOEC (O-SOEC) and the proton-conducting SOEC (H-SOEC) have been actively investigated as next-generation electrolysis technologies that can provide high-energy conversion efficiencies for H2O and CO2 electrolysis to sustainably produce hydrogen and low-carbon fuels, thus providing higher-temperature routes for energy storage and conversion. Current research has also focused on the promotion of SOEC critical components to accelerate wider practical implementation. Based on these investigations, this perspective will summarize the most recent progress in the optimization of electrolysis performance and long-term stability of SOECs, with an emphasis on material developments, technological approaches and improving strategies, such as nano-composing, surface/interface engineering, doping and in situ exsolution. Existing technical challenges are also analyzed, and future research directions are proposed to achieve SOEC technical maturity and economic feasibility for diverse conversion applications.
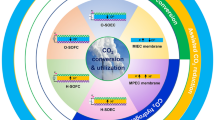
Graphical Abstract
Solid oxide electrolysis cells (SOECs), including oxygen ion-conducting SOEC (O-SOEC) and proton-conducting SOEC (H-SOEC), have been actively investigated as one type of next generation electrolysis technologies with high-energy conversion efficiencies, which provide higher-temperature routes for energy storage and conversion.



Copyright © 2017, American Chemical Society. c and d D* (oxygen solid-state diffusion coefficient) and k* (oxygen surface exchange coefficient) values of current and typical oxygen electrode materials, including La0.6Sr0.4Co0.2Fe0.8O3–δ (LSCF), Ba0.5Sr0.5Co0.8Fe0.2O3–δ (BSCF), Sm0.5Sr0.5Co3–δ (SSC), SrTi0.3Fe0.7O3–δ (STF), SrTi0.3Fe0.63Co0.07O3–δ (STFC), La2NiO4+δ (LNO) and GdBaCo2O5+δ (GBCO). Reprinted with permission from Ref. [11]. Copyright © 2018, Royal Society of Chemistry. Strategies to improve structure. Bulk design: e schematic of a nanocomposite anode consisting of SSC and SDC particles; f EDX mapping of Co (green) and Ce (red) in the SSC-SDC nanocomposite anode; reprinted with permission from Ref. [4]. Copyright 2019 © Nature Publishing Group. g SEM image and h schematic of a honeycomb/vertically aligned LSC-YSZ oxygen electrode. Reprinted with permission from Ref. [35]. Copyright 2018 © Wiley–VCH. Surface/interface engineering: i schematic of an oxygen electrolyte after impregnation (this schematic can also represent electrode materials after the in situ redox exsolution of metal nanoparticles as mentioned below); j EDX element mapping of Ce (green) and Co (red) in the GDC-SSC oxygen electrode; reprinted with permission from Ref. [28]. Copyright 2017 © Elsevier. k HRTEM image of the interfacial region of Au/YSZ; l reaction pathway for OER on the local interface of Au/YSZ, (IS: initial state; TS: transition state; MS: metastable state; FS: final state; atom colors: O: red; Y: purple; Zr: cyan; Au: golden yellow); reprinted with permission from Ref. [14]. Copyright 2019 © Wiley–VCH. m Heterostructured composite electrodes or electrolytes that are usually prepared through chemical processes (this schematic can also represent the common two-phase composite structure) and n SEM image of the local heterointerface of NSC214/NSC113 as an example; o schematic of a heterostructured dense thin-film electrode obtained based on physical processes such as PLD or molecular beam epitaxy (MBE) and p corresponding SEM image, EDX element mapping (green and red are for A (Nd or Sr) and B (Co) sites atoms, respectively) and schematic diagram of the crystal structure. Reprinted with permission from Ref. [36]. Copyright 2018 © Elsevier, and Ref. [37]. Copyright 2020 © Elsevier

Similar content being viewed by others
References
Zheng, Y., Wang, J.C., Yu, B., et al.: A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem. Soc. Rev. 46, 1427–1463 (2017)
Zhao, C.H., Li, Y.F., Zhang, W.Q., et al.: Heterointerface engineering for enhancing the electrochemical performance of solid oxide cells. Energy Environ. Sci. 13, 53–85 (2020)
Duan, C.C., Kee, R., Zhu, H.Y., et al.: Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019)
Shimada, H., Yamaguchi, T., Kishimoto, H., et al.: Nanocomposite electrodes for high current density over 3 A cm−2 in solid oxide electrolysis cells. Nat. Commun. 10, 5432 (2019)
Irvine, J.T.S., Neagu, D., Verbraeken, M.C., et al.: Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 1, 15014 (2016)
Hartvigsen, J., Elangovan, S., Elwell, J., et al.: Oxygen production from Mars atmosphere carbon dioxide using solid oxide electrolysis. ECS Trans. 78, 2953–2963 (2017)
Skafte, T.L., Guan, Z., Machala, M.L., et al.: Selective high-temperature CO2 electrolysis enabled by oxidized carbon intermediates. Nat. Energy 4, 846–855 (2019)
Ni, M., Shao, Z.P.: Fuel cells that operate at 300 °C to 500 °C. Science 369, 138–139 (2020)
Graves, C., Ebbesen, S.D., Jensen, S.H., et al.: Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nat. Mater. 14, 239–244 (2015)
Wu, W., Ding, H.P., Zhang, Y.Y., et al.: 3D self-architectured steam electrode enabled efficient and durable hydrogen production in a proton-conducting solid oxide electrolysis cell at temperatures lower than 600 °C. Adv. Sci. 5, 1870070 (2018)
Zhang, S.L., Wang, H.Q., Lu, M.Y., et al.: Cobalt-substituted SrTi0.3Fe0.7O3–δ: a stable high-performance oxygen electrode material for intermediate-temperature solid oxide electrochemical cells. Energy Environ. Sci. 11, 1870–1879 (2018)
Park, B.K., Zhang, Q., Voorhees, P.W., et al.: Conditions for stable operation of solid oxide electrolysis cells: oxygen electrode effects. Energy Environ. Sci. 12, 3053–3062 (2019)
Zhu, C.L., Hou, S.S., Hu, X.L., et al.: Electrochemical conversion of methane to ethylene in a solid oxide electrolyzer. Nat. Commun. 10, 1173 (2019)
Song, Y.F., Zhou, S., Dong, Q., et al.: Oxygen evolution reaction over the Au/YSZ interface at high temperature. Angew. Chem. 131, 4665–4669 (2019)
Kyriakou, V., Neagu, D., Papaioannou, E.I., et al.: Co-electrolysis of H2O and CO2 on exsolved Ni nanoparticles for efficient syngas generation at controllable H2/CO ratios. Appl. Catal. B: Environ. 258, 117950 (2019)
Wang, W.Y., Gan, L.Z., Lemmon, J.P., et al.: Enhanced carbon dioxide electrolysis at redox manipulated interfaces. Nat. Commun. 10, 1550 (2019)
Li, M., Hua, B., Chen, J., et al.: Charge transfer dynamics in RuO2/perovskite nanohybrid for enhanced electrocatalysis in solid oxide electrolyzers. Nano Energy 57, 186–194 (2019)
Li, W.J., Liu, X.Z., Yu, H., et al.: La0.75Sr0.25Cr0.5Mn0.5O3-δ-Ce0.8Sm0.2O1.9 as composite electrodes in symmetric solid electrolyte cells for electrochemical removal of nitric oxide. Appl. Catal. B: Environ. 264, 118533 (2020)
Lv, H., Liu, T.F., Zhang, X.M., et al.: Atomic-scale insight into exsolution of CoFe alloy nanoparticles in La0.4 Sr0.6 Co0.2 Fe0.7 Mo0.1 O3–δ with efficient CO2 electrolysis. Angew. Chem. Int. Ed. 59, 15968–15973 (2020)
Murphy, R., Zhou, Y.C., Zhang, L., et al.: A new family of proton-conducting electrolytes for reversible solid oxide cells: BaHfxCe0.8-xY0.1Yb0.1O3–δ. Adv. Funct. Mater. 30, 2002265 (2020)
Park, B.K., Scipioni, R., Zhang, Q., et al.: Tuning electrochemical and transport processes to achieve extreme performance and efficiency in solid oxide cells. J. Mater. Chem. A 8, 11687–11694 (2020)
Ding, H.P., Wu, W., Jiang, C., et al.: Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 11, 11 (2020)
Gan, L.Z., Ye, L.T., Ruan, C., et al.: Redox-reversible iron orthovanadate cathode for solid oxide steam electrolyzer. Adv. Sci. 3, 1500186 (2016)
Jun, A., Kim, J., Shin, J., et al.: Achieving high efficiency and eliminating degradation in solid oxide electrochemical cells using high oxygen-capacity perovskite. Angew. Chemie Int. Ed. 55, 12512–12515 (2016)
Liu, S.B., Liu, Q.X., Luo, J.L.: Highly stable and efficient catalyst with in situ exsolved Fe–Ni alloy nanospheres socketed on an oxygen deficient perovskite for direct CO2 electrolysis. ACS Catal. 6, 6219–6228 (2016)
Ye, L.T., Zhang, M.Y., Huang, P., et al.: Enhancing CO2 electrolysis through synergistic control of non-stoichiometry and doping to tune cathode surface structures. Nat. Commun. 8, 14785 (2017)
Kim, S.W., Park, M., Kim, H., et al.: In-situ nano-alloying Pd-Ni for economical control of syngas production from high-temperature thermo-electrochemical reduction of steam/CO2. Appl. Catal. B: Environ. 200, 265–273 (2017)
Joong Yoon, K., Biswas, M., Kim, H.J., et al.: Nano-tailoring of infiltrated catalysts for high-temperature solid oxide regenerative fuel cells. Nano Energy 36, 9–20 (2017)
Li, Y.H., Li, Y., Wan, Y.H., et al.: Perovskite oxyfluoride electrode enabling direct electrolyzing carbon dioxide with excellent electrochemical performances. Adv. Energy Mater. 9, 1803156 (2019)
Hauch, A., Küngas, R., Blennow, P., et al.: Recent advances in solid oxide cell technology for electrolysis. Science 370, 6118 (2020)
Song, Y.F., Zhang, X.M., Xie, K., et al.: High-temperature CO2 electrolysis in solid oxide electrolysis cells: Developments, challenges, and prospects. Adv. Mater. 31, 1902033 (2019)
Gu, X.K., Nikolla, E.: Design of ruddlesden-popper oxides with optimal surface oxygen exchange properties for oxygen reduction and evolution. ACS Catal. 7, 5912–5920 (2017)
Huan, Y., Chen, S.X., Zeng, R., et al.: Intrinsic effects of ruddlesden-popper-based bifunctional catalysts for high-temperature oxygen reduction and evolution. Adv. Energy Mater. 9, 1901573 (2019)
Song, Y.F., Chen, Y.B., Xu, M.G., et al.: A cobalt-free multi-phase nanocomposite as near-ideal cathode of intermediate-temperature solid oxide fuel cells developed by smart self-assembly. Adv. Mater. 32, 1906979 (2020)
Wu, T., Zhang, W.Q., Li, Y.F., et al.: Micro-/nanohoneycomb solid oxide electrolysis cell anodes with ultralarge current tolerance. Adv. Energy Mater. 8, 1802203 (2018)
Zheng, Y., Zhao, C.H., Wu, T., et al.: Enhanced oxygen reduction kinetics by a porous heterostructured cathode for intermediate temperature solid oxide fuel cells. Energy AI 2, 100027 (2020)
Zheng, Y., Li, Y.F., Wu, T., et al.: Oxygen reduction kinetic enhancements of intermediate-temperature SOFC cathodes with novel Nd0.5Sr0.5CoO3−δ/Nd0.8Sr1.2CoO4±δ heterointerfaces. Nano Energy 51, 711–720 (2018)
Zheng, Y., Zhao, C.H., Li, Y.F., et al.: Directly visualizing and exploring local heterointerface with high electro-catalytic activity. Nano Energy 78, 105236 (2020)
Zheng, Y., Li, Y.F., Wu, T., et al.: Controlling crystal orientation in multilayered heterostructures toward high electro-catalytic activity for oxygen reduction reaction. Nano Energy 62, 521–529 (2019)
Schmitt, R., Nenning, A., Kraynis, O., et al.: A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 49, 554–592 (2020)
Zhou, Y.J., Zhou, Z.W., Song, Y.F., et al.: Enhancing CO2 electrolysis performance with vanadium-doped perovskite cathode in solid oxide electrolysis cell. Nano Energy 50, 43–51 (2018)
Neagu, D., Tsekouras, G., Miller, D.N., et al.: In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 5, 916–923 (2013)
Nishihata, Y., Mizuki, J., Akao, T., et al.: Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418, 164–167 (2002)
Kyriakou, V., Neagu, D., Zafeiropoulos, G., et al.: Symmetrical exsolution of Rh nanoparticles in solid oxide cells for efficient syngas production from greenhouse gases. ACS Catal. 10, 1278–1288 (2020)
Kim, S., Lee, S., Kim, J., et al.: Self-transforming configuration based on atmospheric-adaptive materials for solid oxide cells. Sci. Rep. 8, 17149 (2018)
Park, S., Kim, Y., Han, H., et al.: In situ exsolved Co nanoparticles on ruddlesden-popper material as highly active catalyst for CO2 electrolysis to CO. Appl. Catal. B-Environ. 248, 147–156 (2019)
Deka, D.J., Gunduz, S., Fitzgerald, T., et al.: Production of syngas with controllable H2/CO ratio by high temperature co-electrolysis of CO2 and H2O over Ni and Co- doped lanthanum strontium ferrite perovskite cathodes. Appl. Catal. B: Environ. 248, 487–503 (2019)
Cho, A., Ko, J., Kim, B.K., et al.: Electrocatalysts with increased activity for coelectrolysis of steam and carbon dioxide in solid oxide electrolyzer cells. ACS Catal. 9, 967–976 (2019)
Zhu, J.X., Zhang, W.Q., Li, Y.F., et al.: Enhancing CO2 catalytic activation and direct electroreduction on in situ exsolved Fe/MnOx nanoparticles from (Pr, Ba)2Mn2−yFeyO5+δ layered perovskites for SOEC cathodes. Appl. Catal. B: Environ. 268, 118389 (2020)
Neagu, D., Oh, T.S., Miller, D.N., et al.: Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 6, 8120 (2015)
Gao, R., Jain, A.C.P., Pandya, S., et al.: Designing optimal perovskite structure for high ionic conduction. Adv. Mater. 32, 1905178 (2020)
Fop, S., Skakle, J.M.S., McLaughlin, A.C., et al.: Oxide ion conductivity in the hexagonal perovskite derivative Ba3MoNbO8.5. J. Am. Chem. Soc. 138, 16764–16769 (2016)
Duan, C., Tong, J., Shang, M., et al.: Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015)
Draber, F.M., Ader, C., Arnold, J.P., et al.: Nanoscale percolation in doped BaZrO3 for high proton mobility. Nat. Mater. 19, 338–346 (2020)
Acknowledgements
The authors would like to acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), the University of Waterloo and the Waterloo Institute for Nanotechnology.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, Y., Chen, Z. & Zhang, J. Solid Oxide Electrolysis of H2O and CO2 to Produce Hydrogen and Low-Carbon Fuels. Electrochem. Energ. Rev. 4, 508–517 (2021). https://doi.org/10.1007/s41918-021-00097-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-021-00097-4