Abstract
The versatility of supramolecular design in creating biomaterials and drug delivery devices for applications in medicine has gained considerable traction in recent years. The design of peptide-based self-assembling materials is one example of a highly useful and biomimetic approach to the generation of supramolecular biomaterials. One exciting area where designed supramolecular biomaterials created from peptides have demonstrated promise is in the field of immunoengineering. Specifically, peptide-based biomaterials have been used in several different contexts to modify the host immune system through the controlled release of active signaling proteins, pharmaceutical agents, or gasotransmitters. In a separate approach, this class of materials has emerged as a powerful immune-modulating strategy that can enlist the adaptive immune system in mounting a cellular or humoral immune response to a presented epitope or antigen. The ease with which these materials are synthesized, their alignment with injection-based procedures, their low toxicity, and their rapid biodegradation make these useful materials for application in immunoengineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
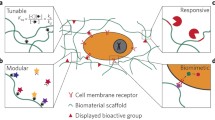
The applied use of supramolecular chemistry underlies a powerful approach to the creation of functional materials with broad-reaching applications1,2,3,4,–5. The supramolecular motifs within these materials, typically comprised of hydrogen bonds, hydrophobic interactions, and π–π interactions, lead to a number of emergent properties as a result of their dynamics which are, typically, governed by equilibrium thermodynamics2. In particular, the use of supramolecular design to create biomaterials affords platforms that are highly modular, tunable, dynamic, and responsive to small changes in their environments6. The properties underlying these materials are typically rooted in their specific yet reversible molecular-level interactions. Supramolecular biomaterials can be broadly classified into two specific categories. The first category includes biomaterials prepared by dynamic crosslinking or chain extension of oligomeric building blocks to realize percolated three-dimensional networks, made possible by supramolecular affinity interactions serving as effective “crosslinking” interactions7,8,9,10,–11. The second category includes non-covalent interactions such as biomaterials formed from entanglement of one-dimensional stacks of materials that arise from an axis of non-covalent interactions, leading to structures with lengths many orders of magnitude greater than their widths. These materials in some ways resemble traditional polymers, with the rigidity and high persistence length of these one-dimensional assemblies enabling entanglement and bundling into percolated material networks and hydrogels.
The most extensively used supramolecular biomaterials of the category arising from one-dimensional structures are prepared from self-assembly of peptidic building blocks into stacked one-dimensional nanostructures (Fig. 1)12,13,14,15,16,–17. The filamentous nanostructures resulting from this assembly affords a versatile platform with which to create physically entangled network materials and bioactive filaments3. As a building block, peptides are attractive for their versatility, ease of synthesis, vast sequence space, cost-effectiveness, precise selectivity, biocompatibility, and typical biodegradability. In addition, peptides play an important role in various cellular and subcellular biological processes. Such properties make peptides an important functional biomaterial and have led to the investigation of peptides and peptide-based biomaterials in various biomedical applications such as drug delivery, tissue engineering, wound healing, and immunology3, 17. Peptide-based supramolecular biomaterials are also highly adaptive and responsive, and can reconfigure or self-heal in response to external stimuli18,19,20,–21. These features can be further augmented with the use of biocatalytic processes that leverage enzymes as an actuator of assembly state and morphology14, 15, 22,23,24,25,–26.
There is rapidly growing interest in the use of engineered biomaterials in the context of immunoengineering—that is, using materials in a functional way to actively augment or stimulate an immune response27,28,29,–30. Supramolecular peptide biomaterials have been included in this body of work. Some distinct and beneficial features of peptide-based supramolecular biomaterials for use in immunoengineering include modularity, multivalency, and spatiotemporal control of nanoscale morphology28. This is added to diverse sequence-specific control over a presented signal or stimulus. In this brief review, we will highlight different applications for supramolecular peptide biomaterials in immunoengineering. We will discuss two primary routes by which an immune response can be leveraged using this class of materials. In the first case, immunomodulation is achieved through classical drug delivery principles in the release of active small molecules or signaling proteins that govern an immune response. In the second case, the high-density presentation of immune-recognized sequences on supramolecular peptide biomaterials is used to elicit an immune response in a manner reminiscent of vaccination. We will discuss the design and use of these classes of materials, and their therapeutic relevance in the context of the emerging field of immunoengineering.
2 Immunomodulation Through Controlled Release
One classical use for peptide-based supramolecular biomaterials is as percolated networks for the encapsulation and release of a therapeutic payload. This is typically achieved through two mechanisms: (1) the passive incorporation of a therapeutic molecule within the network through entrapment or partitioning, or (2) controlled incorporation of a therapeutic molecule through covalent attachment by way of labile linkages. Here, we will highlight both mechanisms used in the delivery of signaling proteins, anti-inflammatory drugs, and signaling gases from peptide-based supramolecular biomaterials. In each case, the molecule released is responsible for modulating an immune response. This includes both pro-inflammatory and anti-inflammatory function. We describe different technologies to control release of payload and the applications possible for such a material.
2.1 Cytokine Delivery
Cytokines, a broad class of small proteins (approximately 5–20 kDa), play a vital role in various physiological processes including regulation of immune and inflammatory responses31, 32. They are also important in cell signaling and serve as immune-modulating agents. A number of cytokines are involved in components of an immune response, including those from the family of interleukins, lymphokines, interferons (IFNs), and tumor necrosis factor (TNF)33,34. Because of their role in directing both innate and adaptive immune response, the controlled release of these cytokines has been explored from a number of different materials35, 36. Supramolecular biomaterials prepared from peptides have utility in the controlled release of such signaling proteins. In this direction, the tunable properties and chemical features of peptide-based supramolecular materials offer control of network properties which may be altered to dictate release kinetics of the encapsulated protein. For example, controlled and sustained release of cytokines from supramolecular peptide materials can be dictated by surface charge in the assembled peptide nanostructures. Accordingly, the materials are able to encapsulate and release a number of cytokines from different peptide hydrogel scaffolds with different isoelectric points and net charges35. The release of cytokines from these materials based upon the overall charge of the peptides serves as an effective modulator for the release and functional activity of cytokines by controlling the physicochemical environment in the material35. Beyond passive encapsulation of such signaling proteins, the high density display of peptides on the surface of these materials enables presentation of peptide-binding groups, such as those derived from phage display, with affinity for a protein of interest37, 38. A similar approach has demonstrated capture of active signaling molecules via presentation of heparin sulfate39. By leveraging molecular affinity, the rate at which the bound protein in each of these cases is released may be extended, and the protein may also serve as an active cue in its presentation on the surface of the material.
2.2 Delivery of Signaling Gases
The role of low concentrations of soluble signaling gases, known as gasotransmitters, in dictating function of cells and tissues has been increasingly appreciated. Many such signaling gases, including carbon monoxide (CO), hydrogen sulfide (H2S), and nitric oxide (NO), function on a protein level in contributing to anti-inflammatory redox signaling, and as such play an important role in immunomodulation40. The best-known gasotransmitter, NO, acts as a defense molecule against infection while also regulating the function of immune cells. Supramolecular peptide biomaterials have been used for the encapsulation and release of small molecule NO donors, demonstrating prevention of inflammation-mediated neointimal hyperplasia in an injured blood vessel41. In this example, NO was released from the peptide hydrogel over 4 days, a significant extension in release when compared to the free donor molecules. An alternate approach demonstrated enzyme-triggered release of NO from a β-galactosidase-responsive caged NO donor encapsulated in a peptide material42 (Fig. 2a–c). This NO-releasing supramolecular biomaterial was evaluated for treatment of myocardial infarction in conjunction with adipose-derived mesenchymal stem cells to facilitate the release of NO at the site of injury43. Building on work to passively encapsulate small-molecule NO donors, NO donors have been instead covalently incorporated in the structure of supramolecular peptide materials. In one example, a systemically administered supramolecular peptide nanofiber targeted to bind to sites of injured blood vessels was further modified demonstrating prevention of restenosis following vascular injury44. Another approach to releasing NO payloads was demonstrated by inclusion of multiple nitric oxide donors onto the lysine side chains of a self-assembled peptide material45. NO was released from this peptide matrix over a period of 1 month, promoting endothelial cell proliferation and limiting smooth muscle cell proliferation. This material has been further explored in conjunction with a strategy to treat type 1 diabetes46.
Delivery of signaling gases: enzyme-assisted delivery of NO, including a chemical structure of the naphthalene-appended peptide amphiphile (PA) containing sugar-caged NO donor, b transmission electron microscopy (TEM) of 5% hydrogel of this material, and c NO release kinetics of the hydrogel with different concentrations of β-galactosidase. CO delivery via Ru-based donor, including d Chemical synthesis of Ru-based CO delivering peptide amphiphile, e CO release kinetics from the peptide amphiphile in sol and gel states, and f SEM image of the peptide amphiphile at gel state. H2S release from peptide amphiphile, including g Chemical structure of S-aroylthiooxime-appended peptide amphiphile, h TEM image of the SATO-appended peptide, and i H2S release kinetics from the peptides in ‘gel’ and ‘sol’ state. Figures are adapted and modified with permission from40,42, 47,48.
The use of supramolecular peptides has extended to the release of other immune-modulating signaling gases as well. This has included the release of CO through the use of a ruthenium-based donor appended to an amino acid side chain on a self-assembling peptide47. The hydrogel state of this material prolongs carbon monoxide release and affords anti-inflammatory protective effects on cells exposed to oxidative stress. In another approach, self-assembling peptides were demonstrated for the release of H2S by modifying a peptide gelator with an S-aroylthiooxime (SATO), which releases H2S when triggered by a free thiol, such as physiologic concentrations of soluble cysteine48. Again, the ability of this material to form supramolecular hydrogels prolonged the release of H2S from a cytocompatible hydrogel.
2.3 Delivery of Small Drug Molecules
Peptide-based supramolecular biomaterials afford the ability to deliver a number of hydrophobic small molecule drugs through passive adsorption or encapsulation. For example, the anti-oxidant and anti-inflammatory drug curcumin has been encapsulated into hydrophobic regions of supramolecular peptides49. The use of supramolecular peptide biomaterials for the release of anti-inflammatory drugs through rupture of labile covalent bonds is also an approach that has been used to ensure localized and sustained release of these small molecule drugs. For example, appendage of the anti-inflammatory drug nabumetone via a hydrazone linkage facilitates prolonged release of the small molecule drug in hydrogels prepared from self-assembling peptides50 (Fig. 3). This approach of using hydrazones to extend drug release from a supramolecular peptide material can be further augmented in molecular design of gelator molecules. As such, the position of the appended drug on a supramolecular peptide has been found to dictate the release kinetics51. This arises as a result of differences in the relative degree of solvation in different regions of assembled peptide nanostructures. This approach was also used to control the release of the common anti-inflammatory drug dexamethasone from supramolecular peptide hydrogels52 (Fig. 3d–f). In this case, supramolecular hydrogels led to a prolonged zero-order release of the drug. When applied in an animal model of local inflammation arising from implantation of a biomedical device, the use of this material mitigated inflammation locally while not altering immune response at a tissue site that was 1–2 cm away in the same animal. Self-assembling prodrugs have also been created from anti-inflammatory drugs using these as a hydrophobic driving force with covalent attachment to a peptide-based gelator53. In one exciting example of this approach, ketoprofen was used in the prodrug design, and supramolecular assembly of this material resulted in its retention following intra-articular injection into a joint cavity, with implications in use for treating conditions such as rheumatoid arthritis54.
Controlled and sustained delivery of small drug molecules from peptide biomaterials: Release of nabumetone, including a chemical structure of nabumetone appended peptide amphiphile (PA), b scanning electron microscopic (SEM) image of Nabumetone-PA, and c release profile of nabumetone from the PA. Release of dexamethasone (Dex), including d chemical structures of Dex-PA and control PA, and E–F) Quantification and inflammation in mice following subcutaneous injection of polystyrene microparticles delivered within Dex-PA gel and control PA, as determined by measuring luminescence from the luminol imaging method to visualize reactive oxygen species. Figures adapted with permission from 50, 52.
3 Vaccination and Immunoengineering
Another strategy to modulate the immune system using materials would seek to engineer a defined immune response using a material stimulus. The innate immune system is typically a first line of defense, offering some protection against infectious agents that can be recognized due to common features among pathogens. The adaptive immune system offers a significantly more complex, nuanced, and specific response in a manner that includes both an ability to learn and adapt to new pathogens, and also form memory to enable a response to future insult by these same pathogens27,55. Traditional vaccination leverages adaptive immunity so that the system is primed for future exposure to the same or similar pathogen. The nature of an adaptive immune response can include the production of antibodies—known as a humoral response—and/or generation of T-lymphocytes—known as a cellular response27, 55, 56. Vaccine technologies are a modern marvel in medical practice, directly saving nearly 6 million lives each year57. Typically, vaccines are preparatory, or prophylactic, in that they are administered prior to pathogen exposure to prime the immune system. There are three main classes used clinically. First, live, attenuated vaccines contain the active infectious agent that has been modified for reduced virulence. While this class of vaccines is highly effective at eliciting both humoral and cellular immune responses, this vaccine cannot be safely administered to patients with weakened immune systems56, 58. Second, inactivated vaccines contain inactivated infectious agents making these safer for patients with weakened immune systems, but often induce a reduced immune response and may require multiple doses to be effective55, 59,60,–61. Third, subunit vaccines contain only the specific antigens derived from the pathogen and not the entire infectious agent, inciting immune recognition and response through exposure to recognized components of the pathogen while reducing side effects arising from other components of the pathogen55,56, 58, 62. It should be noted that virtually all of these vaccination approaches require some adjuvant (typically aluminum salts) to stimulate a heightened immune response and make these vaccines effective63, 64. Although recent advancements have improved the efficacy and use of such adjuvants, there remains a need for new approaches to vaccine development65. Supramolecular peptide biomaterials offer one new technology that has been explored in this regard17,66. These considerations for both design and desired biological function offer many opportunities for supramolecular peptide biomaterials (Fig. 4).
Top Panel: adaptive immunity can be controlled by tuning different properties of supramolecular peptide materials, such as morphology, net charge, multivalency, presentation of cell-specific epitopes, and inclusion of adjuvanting agents; bottom panel: adaptive immunity is conveyed through various cellular and humoral responses, which can be engineered for desired immunomodulation.
3.1 Supramolecular Assembly Morphologies
One of the primary factors influencing efficacy of supramolecular vaccines is the morphology of the resultant supramolecular assemblies28,29,61, 66,67,–68. Depending on the amino acid sequence of the peptidic subunits, these macromolecular assemblies can be engineered to produce nanostructures of varying shape ranging from spheres to long filamentous nanofibers69, 70. At sub-gelation concentrations, nanoparticles have been observed to clear quickly from circulation through lymph drainage or macrophage degradation, and produce a milder immune response and less subsequent immune memory. Nanofibers, however, have increased retention and prompt an elevated immune response, often without the need for additional adjuvants28, 66,67,–68,71. At higher concentrations, these fibrillar assemblies entangle to form a hydrogel mesh. These injectable materials facilitate a more stable stimulus within tissue and enable retention of a vaccine signal at a specific site3, 29,56. Supramolecular peptide vaccines offer versatility in controlled design of multivalent systems57,72,73. More complex designs have considered the physical arrangement of presented antigens on the exterior of infectious agents in designing biomimetic assemblies to further increase effectiveness of the immune response elicited by these materials.
3.2 Surface Charge Effects on Immunomodulation
The surface charge of peptidic supramolecular assemblies also is known to dramatically influence the performance of these materials as vaccines. Regarding antigen-presenting cells (APCs), positively charged peptide assemblies have been found to raise a robust immune response, often without the need for adjuvants. While negatively charged peptide assemblies have been found to elicit little or no immune response, this effect is thought to arise due to the general negative average charge present on the APC cell surface74. As such, negatively charged materials are less likely to interact with these cells as a result of electrostatic effects75. Beyond this phenomenon, it is generally observed that the absolute value of the supramolecular assembly zeta potential, rather than the specific positive or negative charges, directs cellular and humoral responses to the exogenous peptidic material. In contrast, neutral materials often display reduced cellular biocompatibility due to disruption of lipid membranes76,77.
3.3 Adjuvant-Free Design
As traditional vaccines were developed, a balance between immunogenicity and safety was required. Live, attenuated vaccines offered certain benefits, but with attendant risks to patient safety. Adjuvants have thus been a hallmark of clinical vaccination practice, serving to heighten the immunogenicity of the vaccine; these are, however, also associated with an inflammatory response27, 56, 68. The underlying mechanisms of many adjuvants are not fully elucidated, limiting their use and further engineering or optimization of these compounds58. Supramolecular peptide assemblies, however, have been found to elicit a complex and robust immune response without the addition of additional adjuvants. It has been shown that assembly aspect ratio and surface multivalency are the primary drivers of this effect; yet supramolecular vaccines at the same time do not elicit the severe inflammation response associated with traditional adjuvants28, 60, 61, 66, 71, 72. Additional design features have also been shown to improve immunogenicity, such as by lipidation of peptidic assemblies55, 59.
3.4 Presentation of Cell-Recognition Sequences
To further enhance and tune the immune response to a supramolecular vaccine for immunomodulation, the ability to control interactions between vaccine peptide assemblies and specific immune cells has been explored. These peptide assemblies are designed to facilitate interaction with and recruitment of T-helper (CD4+) or cytotoxic (CD8+) T-cells. To involve CD4+ T-helper cells in an immune response, the universal CD4+ peptide epitope sequence known as PADRE (aKXVAAWTLKAa, where X is cyclohexylalanine and a is d-alanine) has shown success in increasing the immune response to certain antigens. To achieve a more cytotoxic response, the presentation of model T cell epitopes has been shown to selectively promote increased CD8+ T cell involvement in the immune response67.
3.5 Expanding Vaccine Applications
In addition to traditional prophylactic approaches, supramolecular peptide vaccines can be expanded to immunotherapy, such as in the treatment of cancer. By engineering peptide assemblies with cancer-associated antigens along with sensitizing or pro-inflammatory cues, the immune system can be programmed to detect and destroy cancer cells27, 56, 58, 78. Initial trials using this concept have shown increased survival in mouse cancer models27, 79,80. Another exciting direction is in using supramolecular peptide vaccines to address autoimmune diseases or transplant rejection by promoting immune tolerance. By presenting “self” antigens on peptide assemblies along with anti-inflammatory, tolerogenic cues, T-lymphocyte function can be evolved from cytotoxic to regulatory phenotypes, helping to address autoimmune diseases or immune rejection by improved immune tolerance27, 58, 73,81.
4 Conclusions
Supramolecular peptide biomaterials afford numerous opportunities to interface with, and modulate, the immune system. This has been manifested in a number of different applications, ranging from the delivery of active drugs or signaling molecules to structural and/or functional mimicry of pathogens in promoting an adaptive immune response. In the delivery of drugs or signaling molecules, supramolecular peptide biomaterials afford a variety of structural features that contribute to their function. Many anti-inflammatory drugs are also hydrophobic, and these materials offer hydrophobic environments within aggregated structures to facilitate partition-driven preferential uptake and controlled release. This can be further augmented by covalently attaching drugs to the extensive available surface area in these nanostructures by way of labile bonds for added control over drug localization and release kinetics. Many of the signaling gases discussed arise from donor molecules that have very short half-lives in water. Using supramolecular peptide biomaterials to deliver these donor molecules, these materials afford environments with reduced availability and/or mobility of water to stabilize the donor molecules and prolong release of active drug. In delivering larger cytokines, supramolecular peptide biomaterials facilitate physical entanglement of one-dimensional nanostructures into a percolated mesh. Such a morphology is able to entrap signaling proteins and control their release.
In facilitating immunomodulation using this class of materials to promote an adaptive immune response, a critical feature is the high-density display of epitopes of interest on the surface of these same one-dimensional nanofibers. This had led to numerous exciting results in the context of adjuvant-free vaccination, with possibilities for disease-tailored or personalized vaccine platforms displaying specific epitopes to prompt an engineered immune response. There are a number of inherent advantages to this approach. The ability to rapidly synthesize and purify small molecule peptides using automated solid-phase synthesis offers an accelerated route to vaccine production, contrasting with a timeline on the order of many months to raise traditional vaccines. Moreover, introducing patient- or disease-specific antigens is relatively facile with the modular, mix-and-match nature of many of these supramolecular peptide systems. The ability to achieve immunity without adjuvant is a benefit that cannot be emphasized enough, allowing a robust immune response without the inflammation and possibility for a sensitivity or allergic reaction to the adjuvant.
In the context of translation, the class of peptide-based supramolecular biomaterials offers a number of benefits. This approach to generating biomaterials yields three-dimensional structures that maintain the ability to be injected by shear-thinning and self-healing mechanisms as well as rapid in situ assembly triggered by physiologic cues such as pH or ionic strength. Moreover, peptide synthesis is a facile, high-yielding, and scalable process. The clinical feasibility of this is evident in a variety of pharmaceuticals that have come to market in recent years based on peptide inhibitors. The degradability of these materials into amino acids is also predictable, and not expected to result in toxic products. As such, we share in the great excitement for the use of this class of materials for applications in immunoengineering through drug delivery and immune modulation.
Moving forward, there are many interesting directions where supramolecular biomaterials may realize an impact in the context of immune response. Such success is likely to be predicated on the advancement of companion fields, such as more accurate disease diagnostics and prognostic abilities from the power of “-omics” disciplines. With the increase in incidence of various cancers presenting a present and growing health challenge, rapid disease characterization may be paired with the relative ease of preparation for synthetic, abiologic materials to provide patient-specific cancer vaccine formulations within days of a diagnosis. Similarly, the increased prevalence of autoimmune diseases suggests a possibility for new paradigms of tolerogenic reprogramming leveraging the controllable and tunable properties of synthetic biomimetic materials.
References
Aida T, Meijer EW, Stupp SI (2012) Functional supramolecular polymers. Science 335:813–817
Webber MJ, Appel EA, Meijer EW, Langer R (2016) Supramolecular biomaterials. Nat Mater 15:13–26
Sahoo JK, VandenBerg MA, Webber MJ (2017) Injectable network biomaterials via molecular or colloidal self-assembly. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2017.11.005
Zhou J, Li J, Du X, Xu B (2017) Supramolecular biofunctional materials. Biomaterials 129:1–27
Webber MJ, Langer R (2017) Drug delivery by supramolecular design. Chem Soc Rev. https://doi.org/10.1039/C7CS00391A
Webber MJ (2016) Engineering responsive supramolecular biomaterials: toward smart therapeutics. Bioeng Transl Med 1:252–266
Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA (2015) Cucurbituril-based molecular recognition. Chem Rev 115:12320–12406
Hu Q-D, Tang G-P, Chu PK (2014) Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: from design to applications. Acc Chem Res 47:2017–2025
Appel EA, del Barrio J, Loh XJ, Scherman OA (2012) Supramolecular polymeric hydrogels. Chem Soc Rev 41:6195–6214
Mozhdehi D, Ayala S, Cromwell OR, Guan Z (2014) Self-healing multiphase polymers via dynamic metal-ligand interactions. J Am Chem Soc 136:16128–16131
Rodell CB, Mealy JE, Burdick JA (2015) Supramolecular guest-host interactions for the preparation of biomedical materials. Bioconjug Chem 26:2279–2289
Gazit E (2007) Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem Soc Rev 36:1263–1269
Cui H, Webber MJ, Stupp SI (2010) Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Pept Sci 94:1–18
Fleming S, Ulijn RV (2014) Design of nanostructures based on aromatic peptide amphiphiles. Chem Soc Rev 43:8150–8177
Du X, Zhou J, Shi J, Xu B (2015) Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev 115:13165–13307
Hendricks MP, Sato K, Palmer LC, Stupp SI (2017) Supramolecular assembly of peptide amphiphiles. Acc Chem Res 50:2440–2448
Acar H et al (2017) Self-assembling peptide-based building blocks in medical applications. Adv Drug Deliv Rev 110:65–79
Wang J et al (2016) Trace solvent as a predominant factor to tune dipeptide self-assembly. ACS Nano 10:2138–2143
Pappas CG et al (2015) Transient supramolecular reconfiguration of peptide nanostructures using ultrasound. Mater Horiz 2:198–202
Draper ER, Adams DJ (2016) Photoresponsive gelators. Chem Commun 52:8196–8206
Sahoo JK, Nalluri SKM, Javid N, Webb H, Ulijn RV (2014) Biocatalytic amide condensation and gelation controlled by light. Chem Commun 50:5462–5464
Feng Z, Zhang T, Wang H, Xu B (2017) Supramolecular catalysis and dynamic assemblies for medicine. Chem Soc Rev. https://doi.org/10.1039/C7CS00472A
Zhou J, Li J, Du X, Xu B (2017) Supramolecular biofunctional materials. Biomaterials 129:1–27
Conte MP, Sahoo JKK, Abul-Haija YM, Lau KHA, Ulijn RV (2017) Biocatalytic self-assembly on magnetic nanoparticles. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.7b15456
Zelzer M, Todd SJ, Hirst AR, McDonald TO, Ulijn RV (2012) Enzyme responsive materials: design strategies and future developments. Biomater Sci 1:11–39
Sahoo JK et al (2017) Pathway-dependent gold nanoparticle formation by biocatalytic self-assembly. Nanoscale 9:12330–12334
Tostanoski LH, Jewell CM (2017) Engineering self-assembled materials to study and direct immune function. Adv Drug Deliv Rev 114:60–78
Wen Y, Collier JH (2015) Supramolecular peptide vaccines: tuning adaptive immunity. Curr Opin Immunol 35:73–79
Eskandari S, Guerin T, Toth I, Stephenson RJ (2017) Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev 110:169–187
Kelly SH, Shores LS, Votaw NL, Collier JH (2017) Biomaterials strategies for generating therapeutic immune responses. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2017.04.009
Hapel AJ, McColl SR (1996) Cytokines in immunology. In: Bittar EE, Bittar N (eds) Principles of medical biology, vol 6. Elsevier, Amsterdam, pp 151–169
Zhang J-M, An J (2007) Cytokines, inflammation and Pain. Int Anesthesiol Clin 45:27–37
Bendtzen K (1988) Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett 19:183–192
Medzhitov R, Janeway CA (1997) Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9:4–9
Gelain F, Unsworth LD, Zhang S (2010) Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J Control Release 145:231–239
Yoo JW, Irvine DJ, Discher DE, Mitragotri S (2011) Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov 10:3499
Smith GP, Petrenko VA (1997) Phage display. Chem Rev 97:391–410
Behanna HA, Donners JJJM, Gordon AC, Stupp SI (2005) Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J Am Chem Soc 127:1193–1200
Rajangam K et al (2006) Heparin binding nanostructures to promote growth of blood vessels. Nano Lett 6:2086–2090
Qian Y, Matson JB (2017) Gasotransmitter delivery via self-assembling peptides: treating diseases with natural signaling gases. Adv Drug Deliv Rev 110–111:137–156
Kapadia MR et al (2008) Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg 47:173–182
Gao J et al (2013) Enzyme-controllable delivery of nitric oxide from a molecular hydrogel. Chem Commun 49:9173–9175
Yao X et al (2015) Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 60:130–140
Kassam HA, Moreira ES, Moyer TJ, Stupp SI, Kibbe MR (2013) Prevention of neointimal hyperplasia with systemic injection of a targeted drug-eluting peptide amphiphile. J Surg Res 179:296–297
Kushwaha M et al (2010) A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials 31:1502–1508
Lim D-J et al (2014) Enhanced MIN-6 beta cell survival and function on a nitric oxide-releasing peptide amphiphile nanomatrix. Int J Nanomed 9:13–21
Matson JB, Webber MJ, Tamboli VK, Weber B, Stupp SI (2012) A peptide-based material for therapeutic carbon monoxide delivery. Soft Matter 8:6689–6692
Carter JM, Qian Y, Foster JC, Matson JB (2015) Peptide-based hydrogen sulphide-releasing gels. Chem Commun 51:13131–13134
Altunbas A, Lee SJ, Rajasekaran SA, Schneider JP, Pochan DJ (2011) Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 32:5906–5914
Matson JB, Stupp SI (2011) Drug release from hydrazone-containing peptide amphiphiles. Chem Commun 47:7962–7964
Matson JB, Newcomb CJ, Bitton R, Stupp SI (2012) Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter 8:3586–3595
Webber MJ, Matson JB, Tamboli VK, Stupp SI (2012) Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials 33:6823–6832
Li J et al (2013) The conjugation of nonsteroidal anti-inflammatory drugs (NSAID) to small peptides for generating multifunctional supramolecular nanofibers/hydrogels. Beilstein J Org Chem 9:908–917
Chen Z et al (2017) Drug-bearing supramolecular filament hydrogels as anti-inflammatory agents. Theranostics 7:2003–2014
Zaman M, Toth I (2013) Immunostimulation by synthetic lipopeptide-based vaccine candidates: structure-activity relationships. Front Immunol 4:318
Luo Z et al (2017) A powerful CD8+ T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv Mater 29:1601776
López-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ (2016) Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J 14:58–68
Moyer TJ, Zmolek AC, Irvine DJ (2016) Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest 126:799–808
Joshi VG, Dighe VD, Thakuria D, Malik YS, Kumar S (2013) Multiple antigenic peptide (MAP): a synthetic peptide dendrimer for diagnostic, antiviral and vaccine strategies for emerging and re-emerging viral diseases. Indian J Virol 24:312–320
Chen J et al (2013) The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 34:8776–8785
Chesson CB et al (2014) Antigenic peptide nanofibers elicit adjuvant-free CD8+ T cell responses. Vaccine 32:1174–1180
Heath WR, Carbone FR (2001) Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol 1:nri35100512
McKee AS, Marrack P (2017) Old and new adjuvants. Curr Opin Immunol 47:44–51
Bastola R et al (2017) Vaccine adjuvants: smart components to boost the immune system. Arch Pharm Res. https://doi.org/10.1007/s12272-017-0969-z
Tohumeken S et al (2017) A modular antigen presenting peptide/oligonucleotide nanostructure platform for inducing potent immune response. Adv Biosyst 1:1700015
Rad-Malekshahi M, Lempsink L, Amidi M, Hennink WE, Mastrobattista E (2016) Biomedical applications of self-assembling peptides. Bioconjug Chem 27:3–18
Wu Y et al (2017) A supramolecular vaccine platform based on α-helical peptide nanofibers. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.7b00561
Mora-Solano C et al (2017) Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials 149:1–11
Sahoo JK, Nazareth C, VandenBerg M, Webber M (2017) Self-assembly of amphiphilic tripeptides with sequence-dependent nanostructure. Biomater Sci. https://doi.org/10.1039/C7BM00304H
Sahoo JK, Pappas CG, Sasselli IR, Abul-Haija YM, Ulijn RV (2017) Biocatalytic self-assembly cascades. Angew Chem Int Ed 56:6828–6832
Rudra JS, Tian YF, Jung JP, Collier JH (2010) A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci 107:622–627
Hudalla GA et al (2013) A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv Healthcare Mater 2:1114–1119
Liu H, Irvine DJ (2015) Guiding principles in the design of molecular bioconjugates for vaccine applications. Bioconjug Chem 26:791–801
Andorko JI, Jewell CM (2017) Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng Transl Med 2:139–155
Wen Y, Waltman A, Han H, Collier JH (2016) Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano 10:9274–9286
Hotaling NA, Tang L, Irvine DJ, Babensee JE (2015) Biomaterial strategies for immunomodulation. Annu Rev Biomed Eng 17:317–349
Newcomb CJ et al (2014) Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat Commun 5:3321
Rad-Malekshahi M et al (2017) Self-assembling peptide epitopes as novel platform for anticancer vaccination. Mol Pharm 14:1482–1493
Lin AY et al (2013) Gold nanoparticle delivery of modified cpg stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS One 8:e63550
Zhu G et al (2016) DNA–inorganic hybrid nanovaccine for cancer immunotherapy. Nanoscale 8:6684–6692
Tostanoski LH et al (2016) Reprogramming the local lymph node microenvironment promotes tolerance that is systemic and antigen specific. Cell Rep. 16:2940–2952
Acknowledgements
MJW acknowledges funding support from the University of Notre Dame through the ‘Advancing our Vision’ initiative. We also acknowledge funding support from the Harper Cancer Institute—American Cancer Society Institutional Research Grant (IRG-14-195-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahoo, J.K., Braegelman, A.S. & Webber, M.J. Immunoengineering with Supramolecular Peptide Biomaterials. J Indian Inst Sci 98, 69–79 (2018). https://doi.org/10.1007/s41745-018-0060-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-018-0060-x