Abstract
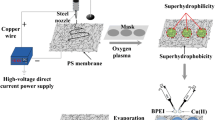
Superwettable surface has broad application prospects in fabricating biosensors due to its significant enrichment effect. Here, we report a polydopamine-based colorimetric superwettable sensor that integrates superhydrophobic–superhydrophilic micropatterns for the determination of hydrogen peroxide (H2O2) and glucose. Dopamine can be oxidized into polydopamine with the addition of horseradish peroxidase (HRP) and H2O2, leading to the deposited spots color change from colorless to black. The concentration of target can be determined by analyzing RGB value using a smartphone software. The superhydrophobic area on the superwettable surface helps capture droplets by confining them to superhydrophilic microwells. After droplet evaporation, the analytes are concentrated in the small superhydrophilic domain, thus greatly enhancing the sensitivity. The experimental results manifested that superwettable sensor is able to detect H2O2 with a broad linear range of 0.25 µmol/L–25 mmol/L and a low limit of detection (LOD) of 0.25 µmol/L by naked eye. For glucose detection, the linear range of the sensor is from 2 µmol/L to 20 mmol/L and LOD is 0.69 μmol/L. The superwettable sensor has been successfully applied in practical samples, including cancerous cells, milk, urine, and human serum samples with acceptable results. This superwettable sensor has several merits, such as high sensitivity, rapid response, and low sample volume in a single microdroplet, and shows great potential in manufacturing portable devices for complex biosensing applications.




Similar content being viewed by others
Data availability
The authors state that all the data supporting the findings are contained within the article and supplementary material.
References
Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Bio. 2007;8:722–8.
Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26(1):1–14.
López-Lázaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252(1):1–8.
Chen W, Cai S, Ren Q-Q, Wen W, Zhao YD. Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst. 2012;137:49–58.
Azad T, Ahmed S. Common milk adulteration and their detection techniques. Int J Food Contam. 2016;3(1):22.
Bopitiya D, Guo S, Hearn MTW, Zhang J, Bennett LE. Formulations of selected energy beverages promote pro-oxidant effects of ascorbic acid and long-term stability of hydrogen peroxide. Food Chem. 2022;388: 133037.
Ivanova AS, Merkuleva AD, Andreev SV, Sakharov KA. Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography. Food Chem. 2019;283:431–6.
Wang Y, Zhou X, Dong W, Zhong Q, Mo X, Li H. Light responsive Fe-Tcpp@ICG for hydrogen peroxide detection and inhibition of tumor cell growth. Biosens Bioelectron. 2022;200: 113931.
Zhang X, Li L, Peng X, Chen R, Huo K, Chu PK. Non-enzymatic hydrogen peroxide photoelectrochemical sensor based on WO3 decorated core–shell TiC/C nanofibers electrode. Electrochim Acta. 2013;108:491–6.
Yue Z, Zhang W, Wang C, Liu G, Niu W. CdS-EePt dimers based photoelectrochemical sensor for detection of H2O2. Mater Lett. 2012;74:180–2.
Rodriguez-Gutierrez R, Ospina NS, Mccoy RG, Lipska KJ, Shah ND, Montori VM. Inclusion of hypoglycemia in clinical practice guidelines and performance measures in the care of patients with diabetes. Jama Intern Med. 2016;176(11):1714–6.
Seaquist ER, Chow LS. Hypoglycemia in diabetes. JAMA. 2017;318(1):31–2.
Nathan DM. Diagnosing diabetes mellitus - best practices still unclear. Nat Rev Endocrinol. 2018;14(10):572–3.
Bergenstal RM. Continuous glucose monitoring: Transforming diabetes management step by step. Lancet. 2018;391(10128):1334–6.
Wang L, Shi XH, Zhang YF, Liu AA, Liu SL, Wang ZG, Pang DW. CdZnSes quantum dots condensed with ordered mesoporous carbon for high-sensitive electrochemiluminescence detection of hydrogen peroxide in live cells. Electrochim Acta. 2020;362: 137107.
Karimi A, Husain SW, Hosseini M, Azar PA, Ganjali MR. Rapid and sensitive detection of hydrogen peroxide in milk by enzyme-free electrochemiluminescence sensor based on a polypyrrole-cerium oxide nanocomposite. Sens Actuators B Chem. 2018;271:90–6.
Li Y, Wang Y, Fu C, Wu Y, Cao H, Shi W, Jung YM. A simple enzyme-free SERS sensor for the rapid and sensitive detection of hydrogen peroxide in food. Analyst. 2020;145(2):607–12.
Zhang R, Zhong Q, Liu Y, Ji J, Liu B. Monodispersed silver-gold nanorods controllable etching for ultrasensitive SERS detection of hydrogen peroxide-involved metabolites. Talanta. 2022;243: 123382.
Zheng DJ, Yang YS, Zhu HL. Recent progress in the development of small-molecule fluorescent probes for the detection of hydrogen peroxide. Trend Anal Chem. 2019;118:625–51.
Żamojć K, Zdrowowicz M, Jacewicz D, Wyrzykowski D, Chmurzyński L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Crit Rev Anal Chem. 2016;46(3):171–200.
Zambrano G, Nastri F, Pavone V, Lombardi A, Chino M. Use of an artificial miniaturized enzyme in hydrogen peroxide detection by chemiluminescence. Sensors. 2020;20(13):3793.
Lu C, Song G, Lin JM. Reactive oxygen species and their chemiluminescence-detection methods. TrAC Trends Anal Chem. 2006;25(10):985–95.
Wang D, Dang X, Tan B, Zhang Q, Zhao H. 3D V2O5-MoS2/rGO nanocomposites with enhanced peroxidase mimicking activity for sensitive colorimetric determination of H2O2 and glucose. Spectrochim Acta A Mol Biomol Spectrosc. 2022;269: 120750.
Liu A, Li M, Wang J, Feng F, Zhang Y, Qiu Z, Chen Y, Meteku BE, Wen C, Yan Z, Zeng J. Ag@Au core/shell triangular nanoplates with dual enzyme-like properties for the colorimetric sensing of glucose. Chin Chem Lett. 2020;31(5):1133–6.
Luo J, Liu R, Zhao S, Gao Y. Bimetallic Fe-Co nanoalloy confined in porous carbon skeleton with enhanced peroxidase mimetic activity for multiple biomarkers monitoring. J Anal Test. 2023;7:53–68.
Sima F, Xu J, Wu D, Sugioka K. Ultrafast laser fabrication of functional biochips: new avenues for exploring 3D micro- and nano-environments. Micromachines. 2017;8(2):40.
Kemmler M, Sauer U, Schleicher E, Preininger C, Brandenburg A. Biochip point-of-care device for sepsis diagnostics. Sens Actuators B Chem. 2014;192:205–15.
Sah V, Baier R. Bacteria inside semiconductors as potential sensor elements: biochip progress. Sensors. 2014;14(6):11225–44.
Lee J-H, Jung H-I. Biochip technology for monitoring posttraumatic stress disorder (PTSD). BioChip J. 2013;7(3):195–200.
Chen X, Zhang L. Review in manufacturing methods of nanochannels of bio-nanofluidic chips. Sens Actuators B Chem. 2018;254:648–59.
Bai H, Wang L, Ju J, Sun R, Zheng Y, Jiang L. Efficient water collection on integrative bioinspired surfaces with star-shaped wettability patterns. Adv Mater. 2014;26(29):5025–30.
Hou Y, Yu M, Chen X, Wang Z, Yao S. Recurrent filmwise and dropwise condensation on a beetle mimetic surface. ACS Nano. 2014;9(1):71–81.
Tsougeni K, Petrou PS, Papageorgiou DP, Kakabakos SE, Tserepi A, Gogolides E. Controlled protein adsorption on microfluidic channels with engineered roughness and wettability. Sens Actuators B Chem. 2012;161(1):216–22.
Songok J, Tuominen M, Teisala H, Haapanen J, Mäkelä J, Kuusipalo J, Toivakka M. Paper-based microfluidics: Fabrication technique and dynamics of capillary-driven surface flow. ACS Appl Mater Interfaces. 2014;6(22):20060–6.
Shi W, Xu T, Xu LP, Chen Y, Wen Y, Zhang X, Wang S. Cell micropatterns based on silicone-oil-modified slippery surfaces. Nanoscale. 2016;8(44):18612–5.
Xu T, Shi W, Huang J, Song Y, Zhang F, Xu LP, Zhang X, Wang S. Superwettable microchips as a platform toward microgravity biosensing. ACS Nano. 2017;11(1):621–6.
Zheng Y, Bai H, Huang Z, Tian X, Nie FQ, Zhao Y, Zhai J, Jiang L. Directional water collection on wetted spider silk. Nature. 2010;463(7281):640–3.
Ueda E, Levkin PA. Emerging applications of superhydrophilic superhydrophobic micropatterns. Adv Mater. 2013;25(9):1234–47.
Wang Y, Liu F, Yang Y, Xu LP. Droplet evaporation-induced analyte concentration toward sensitive biosensing. Mater Chem Front. 2021;5:5639–52.
Xu T, Xu LP, Zhang X, Wang S. Bioinspired superwettable micropatterns for biosensing. Chem Soc Rev. 2019;48(12):3153–65.
Wu T, Xu T, Chen Y, Yang Y, Xu LP, Zhang X, Wang S. Renewable superwettable biochip for mirna detection. Sens Actuators B Chem. 2018;258:715–21.
Zhou M, Fan C, Wang L, Xu T, Zhang X. Enhanced isothermal amplification for ultrafast sensing of SARS-CoV-2 in microdroplets. Anal Chem. 2022;94(10):4135–40.
He X, Xu T, Gao W, Xu LP, Pan T, Zhang X. Flexible superwettable tapes for on-site detection of heavy metals. Anal Chem. 2018;90(24):14105–10.
He X, Fan C, Xu T, Zhang X. Biospired janus silk e-textiles with wet-thermal comfort for highly efficient biofluid monitoring. Nano Lett. 2021;21(20):8880–7.
Xu T, Song Y, Gao W, Wu T, Xu LP, Zhang X, Wang S. Superwettable electrochemical biosensor toward detection of cancer biomarkers. ACS Sensors. 2018;3(1):72–8.
Zhou Z, Wang L, Wang J, Liu C, Xu T, Zhang X. Machine learning with neural networks to enhance selectivity of nonenzymatic electrochemical biosensors in multianalyte mixtures. ACS Appl Mater Interfaces. 2022;14(47):52684–90.
Luo Y, Fan C, Song Y, Xu T, Zhang X. Ultra-trace enriching biosensing in nanoliter sample. Biosens Bioelectron. 2022;210: 114297.
Baker CJ, Deahl K, Domek J, Orlandi EW. Scavenging of H2O2 and production of oxygen by horseradish peroxidase. Arch Biochem Biophys. 2000;382(2):232–7.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30.
Lu Y, Sathasivam S, Song J, Crick CR, Carmalt CJ, Parkin IP. Robust self-cleaning surfaces that function when exposed to either air or oil. Science. 2015;347(6226):1132–5.
Kim CH, Kim B-H, Yang KS. TiO2 nanoparticles loaded on graphene/carbon composite nanofibers by electrospinning for increased photocatalysis. Carbon. 2012;50(7):2472–81.
Lin A, Liu Q, Zhang Y, Wang Q, Li S, Zhu B, Miao L, Du Y, Zhao S, Wei H. A dopamine-enabled universal assay for catalase and catalase-like nanozymes. Anal Chem. 2022;94(30):10636–42.
Zheng D, He Y, Hu N, Wang H. Synthesis and characterization of dopamine-modified Ca-alginate/poly(N-isopropylacrylamide) microspheres for water retention and multi-responsive controlled release of agrochemicals. Int J Biol Macromol. 2020;160:518–30.
Qiang W, Li W, Li X, Chen X, Xu D. Bioinspired polydopamine nanospheres: A superquencher for fluorescence sensing of biomolecules. Chem Sci. 2014;5(8):3018–24.
Liu C, Wang D, Zhang S, Cheng Y, Yang F, Xing Y, Xu T, Dong H, Zhang X. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano. 2019;13(4):4267–77.
Wan X, Song L, Pan W, Zhong H, Li N, Tang B. Tumor-targeted cascade nanoreactor based on metal-organic frameworks for synergistic ferroptosis-starvation anticancer therapy. ACS Nano. 2020;14(9):11017–28.
Fu LH, Wan Y, Qi C, He J, Li C, Yang C, Xu H, Lin J, Huang P. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater. 2021;33(7): e2006892.
Chen TL, Weng HS. A method for the determinations of the activity and optimal pH of glucose oxidase in an unbuffered solution. Biotechnol Bioeng. 1986;28(1):107–9.
Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (22176080) and SRT Program of University of Jinan (Yuhao Li).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Li, J., Li, Y. & Gao, Z. Polydopamine-Based Colorimetric Superwettable Biosensor for Highly Sensitive Detection of Hydrogen Peroxide and Glucose. J. Anal. Test. 7, 118–127 (2023). https://doi.org/10.1007/s41664-023-00252-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-023-00252-4