Abstracts
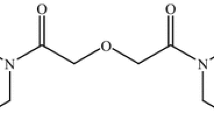
A novel, unsymmetrical diglycolamide, N,N′-dibutyl–N,N′-di(1-methylheptyl)-diglycolamide (DBD1MHDGA), was synthesized. The extraction of rare earth elements (REEs) from a hydrochloric acid medium with DBD1MHDGA was investigated. The results of the extraction experiments indicated that the distribution ratios of RE(III) ions increase with an increase in HCl concentration, atomic number, and extractant concentration. The calculated thermodynamic data show that the extraction process is an exothermic reaction. The organic phase loaded rare earth ions were characterized by infrared spectroscopy. The composition of the extracted complex was determined.








Similar content being viewed by others
References
M.E. Nasab, A. Sam, S.A. Milani, Determination of optimum process conditions for the separation of thorium and rare earth elements by solvent extraction. Hydrometallurgy 106, 141–147 (2011). doi:10.1016/j.hydromet.12.014
D.A. Renata, A.M. Carlos, Study on separation of heavy rare earth elements by solvent extraction with organophosphorus acids and amine reagents. Miner. Eng. 61, 82–87 (2014). doi:10.1016/j.mineng.2014.03.015
S. Radhika, K.B. Nagaphani, K.M. Lakshmi, Solvent extraction an separation of rare-earths from phosphoric acid solutions with TOPS 99. Hydrometallurgy 110, 50–55 (2011). doi:10.1016/j.hydromet.2011.08004
B.C. McLellan, G.D. Corder, A. Golev, S.H. Ali, Sustainability of the rare earths industry. Proc. Environ. Sci. 20, 280–287 (2014). doi:10.1016/j.proenv.2014.03.035
Q. Jia, S.S. Tong, Z.Y. Li et al., Solvent extraction of rare earth elements with mixtures of sec-octylphenoxy acetic acid and bis(2,4,4-trimethylpentyl) dithiophosphinic acid. Sep. Purif. Technol. 64, 345–350 (2009). doi:10.1016/j.seppur.2008.10.024
C.Z. Li, C.F. Yao, S.B. Wang, Z.X. Jia, C.F. He, D. Zhao, G.S. Qin, W.P. Qin, Tm3+/Ho3+ co-doped fluorotellurite microstructure fiber for 2.1 μm lasing. Chin. J Lumin 37, 74–80 (2016). doi:10.3788/fgxb20163701.0074
P.Z. Hu, L.J. Qian, H.L. Wang et al., Extraction of uranium(VI) and thorium(IV) from nitric acid solution by N,N,N′,N′–tetraoctylglutaricamide. Sep. Sci. Technol. 49, 1521–1526 (2014). doi:10.1080/01496395.885531
H. Tong, Y.L. Wang, W.P. Liao et al., Synergistic extraction of Ce(IV) and Th(IV) with mixtures of Cyanex 923 and organophosphorus acids in sulfuric acid media. Sep. Purif. Technol. 118, 487–491 (2014). doi:10.1016/j.seppur.2013.07.039
W.H. Duan, P.J. Cao, Y.J. Zhu, Extraction of rare earth elements from their oxides using organophosphorus reagent complexes with HNO3 and H2O in supercritical CO2. Rare Earths 28, 221–226 (2010). doi:10.1016/S1002-0721(09)60084-3
M.M. Tian, Q. Jia, W.P. Liao, Studies on synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex 301 or Cyanex 302. J. Rare Earths 31, 604–608 (2013). doi:10.1016/S1002-0721(12)60328-7
R. Surampally, N.K. Batchu, L.K. Mannepalli et al., Studies on solvent extraction of Dy(III) and separation possibilities of rare earths using PC-88A from phosphoric acid solutions. J. Taiwan Inst. Chem. Eng. 43, 839–844 (2012). doi:10.1016/j.jtice.2012.04.009
H. Huang, S.D. Ding, N. Liu et al., Extraction of trivalent americium and europium from nitric acid solution with a calixarene-based diglycolamide. Sep. Purif. Technol. 123, 235–240 (2014). doi:10.1016/j.seppur.2013.12.039
J. Ravi, T. Prathibha, K.A. Venkatesan et al., Third phase formation of neodymium (III) and nitric acid in unsymmetrical N,N-di-2-ethylhexyl-N′,N′-dioctyldiglycolamide. Sep. Purif. Technol. 85, 96–100 (2012). doi:10.1016/j.seppur.2011.09.053
G.X. Sun, M. Liu, Y. Cui et al., Synthesis of N,N′-dimethyl-N,N′-dioctyl-3-oxadiglycolamide and its extraction properties for lanthanides. Solvent Extr. Ion Exch. 28, 482–494 (2010). doi:10.1080/07366299.2010.480932
S. Yuji, T. Shoichi, Extraction of actinides(III), (IV), (V), (VI), and lanthanides(III) by structurally tailored diamides. Solvent Extr. Ion Exch. 20, 21–34 (2002). doi:10.1081/SEI-100108822
E.M. Nasab, Solvent extraction separation of uranium(VI)and thorium(IV) with neutral organophosphorus andamine ligands. Fuel 116, 595–600 (2014). doi:10.1016/j.fuel.2013.08.043
D.D. Dicholkar, P. Kumar, P.K. Heer et al., Synthesis of N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide (TODGA) and its steam thermolysis-nitrolysis as a nuclear waste solvent minimization method. Ind. Eng. Chem. Res. 52, 2457–2469 (2013). doi:10.1021/ie302603q
D. Magnusson, B. Christiansen, J.P. Glatz et al., Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate. Solvent Extr. Ion Exch. 27, 26–35 (2009). doi:10.1080/07366290802544726
J. Ravi, K.A. Venkatesan, M.P. Antony et al., Tuning the diglycolamides for modifier-free minor actinide partitioning. J. Environ. Chem. Eng. 1, 690–695 (2013). doi:10.1007/s10967-012-1905-9
V. Chavan, V. Thekkethil, A.K. Pandey et al., Assembled diglycolamide for f-element ions sequestration at high acidity. React. Polym. 74, 52–57 (2014). doi:10.1016/j.reactfunctpolym.2013.10.011
E.A. Mowafy, D. Mohamed, Extraction behavior of trivalent lanthanides from nitric acid medium by selected structurally related diglycolamides as novel extractants. Sep. Purif. Technol. 128, 18–24 (2014). doi:10.1016/j.seppur.2014.03.005
S.L. Gwang, U. Masahito, M. Kouji et al., Separation of major impurities Ce, Pr, Nd, Sm, Al, Ca, Fe, and Zn from La using bis(2-ethylhexyl)phosphoric acid (D2EHPA)-impregnated resin in a hydrochloric acid medium. Sep. Purif. Technol. 71, 186–191 (2010). doi:10.1016/j.seppur.2009.11.020
Y. Cui, Y.Q. Wang, M.P. Pang et al., Effect of diluents on the extraction and separation of Fe(III) and Cu(II) from hydrochloric acid solutions using N,N,N′,N′-tetrabutyl succinamide. Hydrometallurgy 152, 1–6 (2015). doi:10.1016/j.hydromet.2014.11.012
H.T. Chang, M. Li, Z.G. Liu, Y.H. Hu et al., Study on separation of rare earth elements in complex system. J. Rare Earths 28, 116–119 (2010). doi:10.1016/S1002-0721(10)60270-0
F. Xie, T.A. Zhang, D. David et al., A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 56, 10–28 (2014). doi:10.1016/j.mineng.2013.10.021
S.S. Tong, X.W. Zhao, W.H. Zhou, Solvent extraction study of rare earth elements from chloride medium by mixtures of sec-nonylphenoxy acetic acid with Cyanex301 or Cyanex302. Hydrometallurgy 100, 15–19 (2009). doi:10.1016/j.hydromet.2009.09.006
S. Dutta, P.K. Mohapatra, V.K. Manchanda, Separation of 90Y from 90Sr by a solvent extraction method using N,N,N′,N′-tetraoctyl diglycolamide (TODGA) as the extractant. Appl. Radiat. Isot. 69, 158–162 (2011). doi:10.1016/j.apradiso.2010.09.016
Y. Cui, J.H. Yang, G. Yang et al., Effect of diluents on extraction behavior of rare earth elements with N,N,N′,N′-tetrabutyl-3-oxy-glutaramide from hydrochloric acid. Hydrometallurgy 121–124, 16–21 (2012). doi:10.1016/j.hydromet.2012.04.013
G. Yang, D. Ma, Study of 3-oxa-glutaramide extractting rare earth metals from hydrochloric acid system. Thesis, University of Jinan (2010)
P.N. Pathak, L.B. Kumbhare, V.K. Manchanda, Structural effects in N,N-dlalkyl amides on their extraction behavior toward uranium and thorium. Solvent Extr. Ion Exch. 19, 105–126 (2011). doi:10.1081/SEI-100001377
E. Hosten, H.E. Rohwer, Complexation reactions of uranyl with arsenazo III. Anal. Chim. Acta 355, 95–100 (1997). doi:10.1016/S0003-2670(97)81616-9
S.P.K. Donald, H.K. Kean, C.Y.J. Chan, The application of the pitzer equations to 1–1 electrolytes in mixed solvents. Solut. Chem. 14, 635–651 (1985). doi:10.1007/BF00646056
S. Manohar, J. Ananthaswamy, G.J. Atkinson, Application of Pitzer equations for quaternary systems: sodium chloride-sodium nitrate-sodium acetate-water and potassium chloride-potassium nitrate-potassium acetate-water at 25 °C. Chem. Eng. Data. 37, 459–463 (1992). doi:10.1021/je00008a019
A. Shimada, T. Yaita, H. Narita et al., Extraction studies of lanthanide(III) Ions with N, N′-dimethyl-N,N′-diphenylpyridine-2,6-dicarboxyamide (DMDPhPDA) from nitric acid solutions. Solvent Extr. Ion Exch. 22, 147–161 (2004). doi:10.1081/SEI-120030392
M. Anitha, M.K. Kotekar, D.K. Singh et al., Solvent extraction studies on rare earths from chloride medium with organophosphorous extractant dinonyl phenyl phosphoric acid. Hydrometallurgy 146, 128–132 (2014). doi:10.1016/j.hydromet.2014.03.015
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Natural Science Foundation of China (Nos. 21077044, 21171069).
Rights and permissions
About this article
Cite this article
Sun, GJ., Yang, JH., Yang, HX. et al. Extraction study of rare earth elements with N,N′-dibutyl–N,N′-di(1-methylheptyl)-diglycolamide from hydrochloric acid. NUCL SCI TECH 27, 75 (2016). https://doi.org/10.1007/s41365-016-0055-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41365-016-0055-0