Abstract
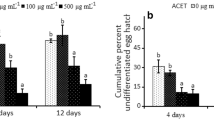
This study shows for the first time the effect of the fermentation filtrate of Myrothecium verrucaria, strain ZW-2, on four different species of plant-parasitic nematodes. Our results suggested that the fermentation filtrate of M. verrucaria inhibited hatching of Meloidogyne incognita and Heterodera glycines eggs. The lowest egg hatching rates of H. glycines and M. incognita were 6.3% and 2.0%, respectively, after 15 days of incubation with a fermentation filtrate of M. verrucaria. A 2-week fermentation filtrate had lethal effects on of M. incognita (second stage juveniles), H. glycines and Bursaphelenchus xylophilus. After 72 h of incubation, the following mortality rates were observed: M. incognita J2s, 100%; Hirschmanniella spp., 8.4%; H. glycines J2s, 82.4%; and B. xylophilus, 96.1%. A pot test showed that the mean suppressive rates of H. glycines after one- and two-week fermentation treatments were 78.8% and 91.2%, respectively. There were 0.8 nematodes per tomato root system at seven days after inoculation (DAI) and 4.4 galls and 933.3 eggs per root system at 30 DAI, which were significantly lower than those of the tap water controls (8.0 nematodes, 31.4 galls and 14,622.5 eggs per root system). Our studies confirmed that the fermentation filtrate of strain ZW-2 has nematicidal effects against three plant-parasitic nematodes.


Similar content being viewed by others
References
Abbas H, Javed N, Khan SA, Kamran M, Atiq M (2015) Exploitation of nematicidal potential of Paecilomyces lilacinus against root knot nematode on eggplant. Int J Veg Sci 22:85–90
Abbas HK, Tak H, Boyette CD, Shier WT, Jarvis BB (2001) Macrocyclic trichothecenes are undetectable in kudzu (Pueraria montana) plants treated with a high-producing isolate of Myrothecium verrucaria. Phytochemistry 58:269–276
Boyette CD, Hoagland RE, Weaver MA, Krishna NR (2008a) Redvine (Brunnichia ovata) and trumpetcreeper (Campsis radicans) controlled under field conditions by a synergistic interaction of the bioherbicide, Myrothecium verrucaria, with glyphosate. Weed Biol Manag 8:39–45
Boyette CD, Weaver MA, Hoagland RE, Stetina KC (2008b) Submerged culture of a mycelial formulation of a bioherbicidal strain of Myrothecium verrucaria with mitigated mycotoxin production. World J Microbiol Biotechnol 24:2721–2726
Colagiero M, Rosso LC, Ciancio A (2018) Diversity and biocontrol potential of bacterial consortia associated to root-knot nematodes. Biol Control 120:11–16
Costa SR, Kerry BR, Bardgett RD, Davies KG (2012) Interactions between nematodes and their microbial enemies in coastal sand dunes. Oecologia 170:1053–1066
Dong HL, Zhou XG, Wang JM, Xu YM, Lu P (2015) Myrothecium verrucaria strain x-16, a novel parasitic fungus to Meloidogyne hapla. Biol Control 83:7–12
Hooper DJ, Southey JF (1970) Extraction of nematodes from plant material. In: Southey JF (ed) Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture, Fisheries and Food Technology, Bull. 2. Her Majesty’s Stationary Office, London, pp 34–38
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne species, including a new technique. Plant Dis Rep 57:1025–1028
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-lópez R, Palomares-rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Kang M, Guo DL, Hu J, Wan B, Zhou Y, Ding LS, Gu YC, Deng Y (2015) Chemical constituents in the broth of Myrothecium verrucaria with agrochemical activities. Nat Prod Res Dev 27:1892–1899
Kerry BR (1989) Fungi as biological control agents for plant parasitic nematodes. In: Whipps JM, Lumsden RDC (eds) Biotechnology of fungi for improving plant growth. Cambridge University Press, Cambridge, pp 153–170
Kiewnick S, Sikora RA (2006) Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Control 38:179–187
Spence KO, Lewis EE (2010) Biopesticides with complex modes of action: direct and indirect effects of DiTera® on Meloidogyne incognita. Nematology 12:835–846
Li J, Todd TC, Lee J, Trick HN (2011) Biotechnological application of functional genomics towards plant-parasitic nematode control. Plant Biotechnol J 9:936–944
Morgan-Jones G, Godoy G, Rodríguez-Kábana R (1981) Research papers: Verticillium chlamydosporium, fungal parasite of Meloidogyne arenaria females. Nematropica 11:115–120
Nagesh M, Javeed S, Ramanujam B, Rangeswaran R (2013) Suitability of soil types for Paecilomyces lilacinus and Pochonia chlamydosporia and their performance against root-knot nematode, Meloidogyne incognita on Lycopersicon esculentum in glasshouse. Indian J Agric Sci 83:826–830
Nguyen LTT, Jang JY, Kim TY, Yu NH, Park AR, Lee S, Bae C, Yeo JH, Hur J, Park HW, Kim J (2018) Nematicidal activity of verrucarin A and roridin A isolated from Myrothecium verrucaria against Meloidogyne incognita. Pestic Biochem Physiol 148:133–143
Nicol JM, Turner SJ, Coyne DL, Nijs LD, Hockland S, Maafi JT (2011) Current nematode threats to world agriculture. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant nematode interactions. Springer, Dordrecht, pp 21–43
Pinkerton JN, Kitner MLC (2006) Effects of biologically-derived products on mobility and reproduction of the root-lesion nematode, Pratylenchus penetrans, on strawberry. Nematropica 36:181–196
Qiu ZQ, Mo AS, He Q, Wu HY, Zhou XB (2016) Root-lesion nematodes on maize in Shandong, China. J Gen Plant Pathol 82:224–227
Rao MS, Kamalnath M, Umamaheswari R, Rajinikanth R, Prabu P, Priti K, Grace GN, Chaya MK, Gopalakrishnan C (2017) Bacillus subtilis IIHR BS-2 enriched vermicompost controls root knot nematode and soft rot disease complex in carrot. Sci Hortic 218:56–62
Rao YS, Israel P (1972) Influence of inoculum density on the final population of root-knot nematode (Meloidogyne graminicola) in rice. Indian J Nemat 2:72–76
Saira M, Rehman A, Gleason ML, Alam MW, Idrees M (2017) First report of Myrothecium verrucaria causing leaf spot of maize in Pakistan. Plant Dis 101:633
Seenivasan N, Lakshmanan PL (2001) Effect of culture filtrates of Pseudomonas fluorescens on rice root nematode Hirschmanniella gracilis. Pestology 11:11–15
Segers R, Butt TM, Kerry BR, Peberdy JF (1994) The nematophagous fungus Verticillium chlamydosporium produces a chymoelastase-like protease which hydrolyses host nematode proteins in situ. Microbiology 140:2715–2723
Shukla M, Kaur P, Kumar A (2016) Molecular aspects of plant-nematode interactions. Indian J Plant Physiol 21:477–488
Szabó M, Csepregi K, Gálber M, Virányi F, Fekete C (2012) Control plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: the role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biol Control 63:121–128
Tan QQ, Wu HY, Jiang SX, Ma HB (2013) Mortality and movement behaviour of Bursaphelenchus xylophilus under different dosages of copper sulphate. Plant Prot Sci 49:98–103
Twomey U, Rolfe RN, Warrior P, Perry RN (2002) Effects of the biological nematicide, DiTera®, on movement and sensory responses of second stage juveniles of Globodera rostochiensis, and stylet activity of G. rostochiensis and fourth stage juveniles of Ditylenchus dipsaci. Nematology 4:909–915
Twomey U, Warrior P, Kerry BR, Perry RN (2000) Effects of the biological nematicide, DiTera®, on hatching of Globodera rostochiensis and G. pallida. Nematology 2:355–362
Walker HL, Tilley AM (1997) Evaluation of an isolate of Myrothecium verrucaria from sicklepod (Senna obtusifolia) as a potential mycoherbicide-agent. Biol Control 10:104–112
Warrior P, Rehberger LA, Beach M, Grau PA, Kirfman GW, Conley JM (1999) Commercial development and introduction of DiTera™, a new nematicide. Pest Manag Sci 55:376–379
Weaver MA, Jin X, Hoagland RE, Boyette CD (2009) Improved bioherbicidal efficacy by Myrothecium verrucaria via spray adjuvants or herbicide mixtures. Biol Control 50:150–156
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31660511, 31460464) and the Special Fund for Agro-Scientific Research in the Public Interest (201503114). We would like to thank American Journal Experts (https://www.aje.com) for editing and reviewing this manuscript for English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H.Y., Zhang, L.Y. & Zhou, X.B. Effects of Myrothecium verrucaria ZW-2 fermentation filtrates on various plant-parasitic nematodes. J Plant Dis Prot 127, 545–552 (2020). https://doi.org/10.1007/s41348-020-00336-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00336-8