Abstract
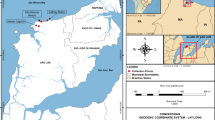
Despite the high abundance and diversity of meiofauna in sandy beach habitats, their patterns of spatial distribution have not been well characterized. This study analyzed the horizontal distribution of the intertidal meiofaunal community in sandy beaches of Todos Santos Bay (TSB), northwestern Mexico, and related it with levels of disturbance. Ten sandy beaches were sampled for meiofauna and sediment along the western coast of TSB at lower, mid, and upper levels of the intertidal. The meiofaunal community included five major groups viz. Amphipoda, Copepoda, Nematoda, Oligochaeta, and Polychaeta, and was mostly dominated by nematodes, regardless of the intertidal level. Meiofauna and nematode abundances differed significantly across the beach slope, increasing towards the upper level; this pattern varied along the shoreline, changing particularly where the beach was heavily modified due to costal development. Multivariate analyses significantly differentiated lower, mid, and upper levels of the intertidal, mostly due to differences in copepod abundance. Sediment grain size significantly differed among intertidal levels (i.e., smaller in the upper intertidal) and was negatively correlated with meiofaunal abundance. Moreover, meiofaunal abundance was negatively impacted by the degree of disturbance as highly urbanized/disturbed beaches of TSB showed lower meiofaunal abundance. As costal development continues to increase, findings from ecological surveys should play a pivotal role in characterizing and monitoring the health status of sandy beaches to aid in their management and conservation.






Similar content being viewed by others
References
Alkemade R, Wielemaker A, Hemminga MA (1992) Stimulation of decomposition of Spartina anglica leaves by the bacterivorous marine nematode Diplolaimelloides bruciei (Monhysteridae). J Exp Mar Bio Ecol 159:267–278
Alves AS, Adão H, Ferrero TJ, Marques JC, Costa MJ, Patrício J (2013) Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: the use of nematodes in ecological quality assessment. Ecol Indic 24:462–475
Ansari ZA, Ingole BS, Parulekar AH (1984) Macrofauna and meiofauna of two sandy beaches at Mombasa, Kenya. Indian J Mar Sci 13:187–189
Austen MC (2004) Natural nematode communities are useful tools to address ecological and applied questions. In: Cook RC, Hunt DJ (eds) Proceedings of the fourth international congress of nematology. Brill, Leiden, pp 1–17
Brown AC, McLachlan A (2002) Sandy shore ecosystems and the threats facing them: some predictions for the year 2025. Environ Conserv 29:62–77
Carver RE (1971) Procedures in Sedimentary petrology. Wiley-Interscience, New York
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/tutorial. PRIMER-E Ltd, Plymouth
Clarke KR, Warwick RM (1994) Similarity-based testing for community pattern: the two-way layout with no replication. Mar Biol 118:167–176
Coull BC (1999) Role of meiofauna in estuarine soft-bottom habitats. Austral Ecol 24:327–343
Dahl E (1952) Aspects of the ecology and zonation of the fauna on sandy beaches. Oikos 4:1–27
Davenport J, Davenport JL (2006) The impact of tourism and personal leisure transport on coastal environments: a review. Estuar Coast Mar Sci 67:280–292
Defeo O, McLachlan A (2005) Patterns, processes and regulatory mechanisms in sandy beach macrofauna: a multi-scale analysis. Mar Ecol Prog Ser 295:1–20
Defeo O, McLachlan A (2013) Global patterns in sandy beach macrofauna: species richness, abundance, biomass and body size. Geomorphology 199:106–114
Defeo O, McLachlan A, Schoeman DS, Schlacher TA, Dugan J, Jones A, Lastra M, Scapini F (2009) Threats to sandy beach ecosystems: A review. Estuar Coast Mar Sci 81:1–12
Dexter DM (1992) Sandy beach community structure: the role of exposure and latitude. J Biogeogr 19:59–66
Díaz-Castañeda V, de León-González JA, Solana-Arellano E (2014) Biodiversity of polychaete assemblages in a highly productive lagoon located in Baja California Sur, México. Proc Biol Soc Wash 127:406–422
Díaz-Castañeda V, Harris LH (2004) Biodiversity and structure of the polychaete fauna from soft bottoms of Bahia Todos Santos, Baja California, Mexico. Deep-Sea Res PT II 51:827–847
Espinosa-Carreón L, Gaxiola-Castro G, Robles-Pacheco JM, Nájera-Martínez S (2001) Temparature, salinity, nutrients and chlorophyll a in coastal waters of sourthern California Bight. Cienc Mar 27:397–422
Félix G, Marenzi RC, Polette M, Netto SA (2016) Landscape visual quality and meiofauna biodiversity on sandy beaches. Environ Manag 58:682–693
Fricke AH, Hennig HK, Orren MJ (1981) Relationship between oil pollution and psammolittoral meiofauna density of two South African beaches. Mar Environ Res 5:59–77
Gheskiere T, Hoste E, Vanaverbeke J, Vincx M, Degraer S (2004) Horizontal zonation patterns and feeding structure of marine nematode assemblages on a macrotidal, ultra-dissipative sandy beach (De Panne, Belgium). J Sea Res 52:211–226
Gheskiere T, Vincx M, Urban-Malinga B, Rossano C, Scapini F, Degraer S (2005a) Nematodes from wave-dominated sandy beaches: diversity, zonation patterns and testing of the isocommunities concept. Estuar Coast Mar Sci 62:365–375
Gheskiere T, Vincx M, Weslawski JM, Scapini F, Degraer S (2005b) Meiofauna as descriptor of tourism-induced changes at sandy beaches. Mar Environ Res 60:245–265
Giere O, Pfannkuche O (1982) Biology and ecology of marine Oligochaeta, a review. Oceanogr Mar Biol 20:173–308
Gingold R, Mundo-Ocampo M, Holovachov O, Rocha-Olivares A (2010) The role of habitat heterogeneity in structuring the community of intertidal free-living marine nematodes. Mar Biol 157:1741–1753
Harriague AC, Misic C, Valentini I, Polidori E, Albertelli G, Pusceddu A (2013) Meio- and macrofauna communities in three sandy beaches of the northern Adriatic Sea protected by artificial reefs. Chem Ecol 29:181–195
Heip C, Vincx M, Vranken G (1985) The ecology of marine nematodes. Oceanogr Mar Biol 23:399–489
Hooge MD (1999) Abundance and horizontal distribution of meiofauna on a northern California beach. Pac Sci 53:305–315
Hourston M, Warwick RM, Valesini FJ, Potter IC (2005) To what extent are the characteristics of nematode assemblages in nearshore sediments on the west Australian coast related to habitat type, season and zone? Estuar Coast Mar Sci 64:601–612
Houston JR (2008) The economic value of beaches: a 2008 update. Shore Beach 76:22–26
INEGI (2015) Estimación de la población a mitad de año por entidad federativa y municipio 2015, Mexico
Jiménez-Pérez LC, Molina-Peralta F, Núñez-Fernández E (1992) Effects of waste waters on benthic macrofauna of sandy beaches in Todos Santos Bay. Cienc Mar 18:35–54
Joint IR, Gee JM, Warwick RM (1982) Determination of fine-scale vertical distribution of microbes and meiofauna in an intertidal sediment. Mar Biol 72:157–164
Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. Mar Biol 42:143–148
Kotwicki L, De Troch M, Urban-Malinga B, Gheskiere T, Wesawski JM (2005) Horizontal and vertical distribution of meiofauna on sandy beaches of the North Sea (The Netherlands, Belgium, France). Helgoland Mar Res 59:255–264
Kotwicki L, Deidun A, Grzelak K, Gianni F (2014) A preliminary comparative assessment of the meiofaunal communities of Maltese pocket sandy beaches. Estuar Coast Mar Sci 150:111–119
Kuk-Dzul JG, Díaz-Castañeda V (2016) The relationship between mollusks and oxygen concentrations in Todos Santos Bay, Baja California, Mexico. J Mar Biol. doi:10.1155/2016/5757198
Ladah LB, Tapia FJ, Pineda J, López M (2005) Spatially heterogeneous, synchronous settlement of Chthamalus spp. larvae in northern Baja California. Mar Ecol Prog Ser 302:177–185
Lercari D, Defeo O (2003) Variation of a sandy beach macrobenthic community along a human-induced environmental gradient. Estuar Coast Mar Sci 58:17–24
Levin LA, Ekau W, Gooday AJ, Jorissen F, Middelburg JJ, Naqvi SWA, Neira C, Rabalais NN, Zhang J (2009) Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6:2063–2098
Maria TF, Vanaverbeke J, Gingold R, Esteves AM, Vanreusel A (2013) Tidal exposure or microhabitats: what determines sandy-beach nematode zonation? a case study of a macrotidal ridge-and-runnel sandy beach in Belgium. Mar Ecol 34:207–217
Maria TF, Vanaverbeke J, Vanreusel A, Esteves AM (2016) Sandy beaches: state of the art of nematode ecology. An Acad Bras Cienc 88:1635–1653
Mateos E, Marinone SG, Parés-Sierra A (2009) Towards the numerical simulation of the summer circulation in Todos Santos Bay, Ensenada, B.C. Mexico. Ocean Model 27:107–112
McLachlan A (1980) Intertidal zonation of macrofauna and stratification of meiofauna on high energy sandy beaches in the Eastern Cape, South Africa. T Roy Soc S Afr 44:213–223
McLachlan A, Brown AC (2006) The ecology of sandy shores, 2nd edn. Academic Press, San Diego
Moffett MD, McLachlan A, Winter PED, De Ruyck AMC (1998) Impact of trampling on sandy beach macrofauna. J Coastal Conserv 4:87–90
Moreno M, Ferrero TJ, Granelli V, Marin V, Albertelli G, Fabiano M (2006) Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuar Coast Mar Sci 66:357–367
Muñoz-Barbosa A, Gutiérrez-Galindo EA, Daesslé LW, Orozco-Borbón MV, Segovia-Zavala JA (2012) Relationship between metal enrichments and a biological adverse effects index in sediments from Todos Santos Bay, northwest coast of Baja California, México. Mar Pollut Bull 64:405–409
Nicholas WL, Hodda M (1999) The free-living nematodes of a temperate, high energy, sandy beach: faunal composition and variation over space and time. Hydrobiologia 394:113–127
Peña-Manjarrez JL, Helenes J, Gaxiola-Castro G, Orellana-Cepeda E (2005) Dinoflagellate cysts and bloom events at Todos Santos Bay, Baja California, México, 1999-2000. Cont Shelf Res 25:1375–1393
Peynador C, Méndez-Sánchez F (2010) Managing coastal erosion: A management proposal for a littoral cell in Todos Santos Bay, Ensenada, Baja California, Mexico. Ocean Coast Manage 53:350–357
Raffaelli DG, Mason CF (1981) Pollution monitoring with meiofauna, using the ratio of nematodes to copepods. Mar Pollut Bull 12:158–163
Rodríguez JG (2004) Community structure of intertidal meiofauna along a gradient of morphodynamic states on an exposed North Sea beach. Sarsia 89:22–32
Rodríguez JG, Lastra M, López J (2003) Meiofauna distribution along a gradient of sandy beaches in northern Spain. Estuar Coast Mar Sci 58:63–69
Rodríguez JG, Lopez J, Jaramillo E (2001) Community structure of the intertidal meiofauna along a gradient of morphodynamic sandy beach types in southern Chile. Rev Chil Hist Nat 74:885–897
Rodríguez-Villanueva V, Martínez-Lara R, Macías Zamora V (2003) Polychaete community structure of the northwestern coast of Mexico: patterns of abundance and distribution. Hydrobiologia 496:385–399
Salvat B (1964) Les conditions hydrodynamiques interstitielles des sédiments meubles intertidaux et la répartition verticale de la faune endogée. C R Acad Sci Paris 259:1576–1579
Schlacher TA, Lucrezi S (2010) Compression of home ranges in ghost crabs on sandy beaches impacted by vehicle traffic. Mar Biol 157:2467–2474
Schlacher TA, Schoeman DS, Dugan J, Lastra M, Jones A, Scapini F, McLachlan A (2008) Sandy beach ecosystems: key features, sampling issues, management challenges and climate change impacts. Mar Ecol 29:70–90
Schlacher TA, Weston MA, Schoeman DS, Olds AD, Huijbers CM, Connolly RM (2015) Golden opportunities: A horizon scan to expand sandy beach ecology. Estuar Coast Mar Sci 157:1–6
Schratzberger M, Gee JM, Rees HL, Boyd SE, Wall CM (2000) The structure and taxonomic composition of sublittoral meiofauna assemblages as an indicator of the status of marine environments. J Mar Biol Assoc UK 80:969–980
Sectur (2002) Agenda 21 para el Turismo Mexicano. Un marco de acción para el desarrollo sustentable de la actividad turística, Mexico
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4:67–69
Sokal RR, Rohlf FJ (1995) Biometry: The principles and practices of statistics in biological research. WH Freeman and Company, New York
Somerfield P, Warwick R (1996) Meiofauna in marine pollution monitoring programmes. A laboratory manual. Directorate of Fisheries Research (MAFF), Lowestoft
StatSoft (2005) STATISTICA (data analysis software system)
Steyaert M, Herman PMJ, Moens T, Widdows J, Vincx M (2001) Tidal migration of nematodes on an estuarine tidal flat (the Molenplaat, Schelde Estuary, SW Netherlands). Mar Ecol Prog Ser 224:299–304
Suguio K (1973) Introdução à sedimentologia. Blücher, São Paulo
Walker SJ, Schlacher TA (2011) Impact of a pulse human disturbance experiment on macrofaunal assemblages on an Australian sandy beach. J Coastal Res 27:184–192
Walters K (1988) Diel vertical migration of sediment-associated meiofauna in subtropical sand and seagrass habitats. J Exp Mar Bio Ecol 117:169–186
Warwick RM, Robinson J (2000) Sibling species in the marine pollution indicator genus Pontonema Leidy (Nematoda: Oncholaimidae), with a description of P. mediterranea sp. nov. J Nat Hist 34:641–662
Zeppilli D, Sarrazin J, Leduc D et al (2015) Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar Biodivers 45:505–535
Acknowledgements
The authors thank Mare Britanicum for funding this work through Lorax Consultores. We thank J. Sandoval-Castillo for help during collecting and processing of samples and G. Rendón-Márquez (Geology Department at CICESE) for assistance with sediment analyses. The authors also acknowledge the insightful comments and suggestions from three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pereira, T.J., Gingold, R., Villegas, A.D.M. et al. Patterns of Spatial Variation of Meiofauna in Sandy Beaches of Northwestern Mexico with Contrasting Levels of Disturbance. Thalassas 34, 53–63 (2018). https://doi.org/10.1007/s41208-017-0038-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-017-0038-x