Abstract
The intensive use of pesticides in agriculture generates a contamination of the environment that must be assessed. Bee products such as honey, widely consumed, are not spared by this considerable contamination that can lead to health risks. Bees and their products (honey, nectar, pollen, etc) are considered as real indicators of environmental health. Indeed, these insects, characterized by a specified body and a high mobility, are considered particularly suitable to keep, with high sensitivity, pesticides with which they come in contact. So the analysis of these insects or of their products as honey has been demonstrated as a good bio indicator of environmental quality. In this study, 18 samples of honey collected from North Lebanon, were assayed for their contamination by 84 pesticides. Multi residue extraction was based on QuEChERS, followed by chromatographic analysis by LC–MS/MS and GC–MS/MS. For gas chromatographic analysis, samples were concentrated by SPME before being injected to the GC column. At first, the method was developed and validated using organic honey, and then the collected samples were analyzed. The limits of detection and quantification as well as the repeatability and the reproducibility of the method have been defined: between 0.1 and 25.2 ng g−1 and between 0.6 to 83.1 ng g−1 for the limits detection and quantification respectively. Furthermore, the repeatability was between 0.2 and 15% and the reproducibility between 0.2 and 18%. Concerning the analyzed samples, residues of many pesticides have been found in each sample. Samples collected from the plain of Akkar and the Byblos area, widely known for their agricultural productions, were the most contaminated by pesticides. In fact, pesticide levels found in honey collected from Akkar and Byblos were 1753.92 and 695.13 ng g−1 respectively. Contrariwise, samples collected from the Cedars of Lebanon had the lowest pesticides concentration with a total concentration of 19.5 ng g−1. In conclusion, this study shows that honey can indicate the specific pollution of a given environment and can be considered as an appropriated sentinel for environmental biomonitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pesticide use has greatly increased in recent decades for their effects on crop protection from the harmful activity of parasites (Gómez-Pérez et al. 2012). In fact, many of these pulverized pesticides are scattered in the environment and can then contaminate water, area, soil and food (Tette et al. 2016). By transporting pollen from flower to flower and from plant to plant, bees play an essential role in environmental biomonitoring (Paradis et al. 2014). These species are then considered as good indicators of environmental contamination by pesticides because their foraging activity leading them to cover large areas and to contact many pollutants including pesticides (Badiou-Bénéteau et al. 2013). Likewise bees, with their specific body composition, are particularly appropriate to keep pesticides with which they come into contact and bring them to the hive, which is contaminated either by pesticides collected by bees or by those carried out by pollen and surface area. In addition, the use of pesticides in the treatment of hives during honey collection is another potential contamination source of honey (Kujawski and Namieśnik 2011).
Therefore, the analysis of honey, known for its curative properties in traditional medicine, and for its wide consumption, seems to be a necessity for the establishment of food surveillance. Furthermore, given the wide range of pesticides that can be applied by farmers, multi-residual analytical methods as QuEChERS should be used (Wiest et al. 2011). The QuEChERS method (Quick, Easy, Cheap, Effective, Rugged and Safe) was developed in 2003 for the determination of multi- class pesticide residues in fruits and vegetables and remains the most efficient extraction method for multi-residues pesticides in all food matrixes including honey (Barakat et al. 2007).
Besides the extraction methods, the detection and separation of pesticides pollutants seems to have a special attention (Tette et al. 2016). In fact, both liquid and gas chromatography coupled with tandem mass spectrometry (LC–MS/MS, GC–MS/MS) showed greater efficiency in the multi-residues analysis of pesticide in honey (Bargańska et al. 2013; Debayle et al. 2008; Rial-Otero et al. 2007).
In this study, a simple method based on QuEChERS extraction, followed by samples concentration by SPME was developed for the simultaneous analysis of 84 volatile and non-volatile pesticides. Organochlorine pesticides, known for their large persistence are also considered in this study. Moreover, the two chromatographic techniques, liquid and gas, coupled with tandem mass spectrometry were used in order to cover the large number of pesticides sought. Once the method developed, 18 samples of honey collected from different regions of northern Lebanon were tested for their contamination by pesticides.
Experimentation
Samples collection
For method development, certified organic honey was purchased from local market and was used for the establishment of ranges.
For sample analyzes, 18 samples collected from four main areas of Northern Lebanon [Cedars, Akkar valley, Batroun and Byblos (in the borderline between northern Lebanon and Mount Lebanon)] were obtained from beekeepers in the period of August–September 2015. Sampling sites are shown in Fig. 1.
Samples were then transported to the laboratory in polypropylene tube and maintained at −15 °C until analysis.
Method development
For the extraction of pesticides, an extraction method developed by Malhat et al. was slightly modified and followed by an SPME purification preceding GC–MS/MS analysis (Malhat et al. 2015).
First 5 g of organic honey were weighed in polypropylene tubes (50 mL) then fortified with a mixture of all analyzed pesticides in order to prepare a calibration range with a concentration varying from 10 to 3000 ng g−1. Fortified samples were kept at 4 °C overnight.
For the extraction, 10 mL of ultrapure water and 10 mL of acetonitrile were added to the fortified samples. Then the tubes were shaken manually, then the salts were added (4 g anhydrous magnesium sulfate, 1 g of sodium chloride, sodium citrate 1 g and 0.5 g sodium citrate sesquihydrate of hydrogen). The mixture was then well shaken and centrifuged at 5000 RPM for 10 min. Therefore, the supernatant was transferred to dPSE tubes containing 1200 mg MgSO4, 400 mg and 400 mg PSA C18. The mixture was shaken well and centrifuged at 5000×g for 10 min. Finally the supernatant obtained, underwent evaporation followed by a re-dissolution in 1 mL ACN solution.
100 μL of the resulting final extract were analyzed by LC–MS/MS, and the remaining 900 μL are diluted to 20 mL with saline water for their extraction by SPME before their injection into the gas chromatographic column for their analysis by GC–MS/MS.
Chromatographic analysis
For non-volatile pesticides analysis, 10 µL of the final extract prepared for LC analysis was injected to the liquid chromatography system (Thermo Scientific), coupled to a tandem mass spectrometry system (TSQ Quantum Access Max equipped with a Hyper Quads Driven).
For maximum separation between pesticides a Macherey-Nägel Nucleodur C18 Pyramid column (150 mm × 3 mm; 3 µm) was used at 25 °C, the chromatographic system was also equipped with an autosampler (Accela Autosampler) and the pump was a Surveyor LC Pump Plus (Thermo Scientific).
Elution was carried out at a flow rate of 0.3 mL min−1 using a linear gradient of ACN/water mobile phase. The gradient started with 30:70 (v/v) for 5 min, followed by 50:50 (v/v) for 6 min, then 80:20 (v/v) for 7 min, to achieve 95:5 (v/v) for 10 min, finally a ratio of 30:70 (v/v) for 8 min is recommended in order to stabilize the column for any new injection.
The sought nonvolatile pesticides were: Pymetrozine, Carbendazim, Chloridazone, Acetamiprid, Nicosulfuron, Thiacloprid, Chlortoluron, Carbetamide, Terbutryn, Spinosade A, Isoproturon, Diuron, Metalaxyl-M, Spinosade D, Dimethenanid-p, Penconazole, Isoxadifen, Tebuconazole, Diflubenzuron, Epoxyconazole, Prothioconazole, Propiconazole, Chlorfenvinphos, Triflusulfuron methyl, Pendimethalin, Cyazofamid, Pyraclostrobine, Diflufenican, Flufenoxuron, Lufenuron.
For volatile pesticides analysis: Pesticides were analyzed by gas chromatography coupled to a tandem mass spectrometer. The GC (Thermo Scientific Trace) was coupled to an ITQTM 700 mass spectrometer and injection was done in splitless mode at 250 °C. Sample injection to the GC was also done, by thermal desorption of the SPME fiber, directly after the injection of 2 μL of N-methyl-N-(t-butyldimethylsilyl) trifluoroacetamide (MTBSTFA) used as derivation agent.
Extracts were injected to a semi-polar capillary column TR50 MS 50% phenyl/50% methylsiloxane (60 m × 0.25 mm internal diameter and 0.25 μm as film thickness).
Helium was used as carrier gas at a flow rate of 1 mL min−1.
Pesticides separation was performed as follow: 50 °C (3 min), 10 °C/min to 220 °C (10 min), 3 °C/min to 250 °C (9 min), and 3 °C/min to finally achieve 300 °C where it was maintained for 22 min.
The sought volatile pesticides were: Captan, Tebutam, Fenpropidine, Buprofezin, Trifloxystrobine, Propachlore, Fenpropimorphe, Propargite, Fenarimol, Boscalid, Alachlore, Ethofumesate, s-Metolachlor, Tebufenpyrad, Propyzamid, Myclobutanil, Bifenthrin, Fenpropathrine, Lindane, Isodrine, Aclonifen, Clomazone, Chloridazon, Cyprodinil, Fluzilazole, Oxyfluorfen, Oxadiazon, Propiconazole, Trifluraline, Chlorothalonil, Procymidon, Chlorpyriphos, Bromoxynil, Diclofop-methyl, Bifenox, Bupirimate, Mepanipyrim, α-HCH, γ-HCH, β-HCH, δ-HCH, Heptachlore epoxyde A, o,p′-DDD, o,p′-DDT, p,p′-DDD, p,p′-DDT, α-endosulfan, Aldrine, Heptachlore, Dieldrine, Hexachlorobenzene, Heptachlore epoxyde B, transchlordane, Cischlordane.
The method was then validated and limits of detection (LOD) and quantification (LOQ) were evaluated as the pesticide concentration that produces a peak signal-to-noise ratio of 3/1 and 10/1, respectively. Repeatability and reproducibility of the method were also determined. All analysis were done in triplicate.
Samples analysis
Once the method developed, all collected samples underwent the same extraction procedure of the developed method cited above. Pesticides residues in all the honey samples were calculated using Xcalibur software.
Results and discussion
Method validation
The method has been validated for its linearity, limits of detection and quantification, repeatability and reproducibility. Linearity was validated by calculating the correlation coefficient R 2 which should be greater than 0.9800 for all sough pesticides. The limits of detection and quantification were graphically calculated; LOD = 3* [min] S/N and LOQ = 10* [min] S/N, where [min] = minimal concentration, S = signal detected at this concentration and N = noise.
Finally, the method has been validated for its repeatability calculated by the intraday analysis of the fortification of three samples with the same amount of pesticide and reproducibility analysis calculated by the inter day of the fortification of three samples with the same amount pesticide to four successive days.
The results showed that R 2 > 0.9850 for all analyzed pesticides, limit of detection was ranged from 0.1 to 25.2 ng g−1 while limit of quantification was from 0.6 to 83.1 ng g−1. Then these limits allow pesticides detection at very low concentrations even lower than 1 ng g−1.
For repeatability and reproducibility, the results showed great repeatability and reproducibility; coefficients of variation of intra- and inter days studies were respectively between 0.2 and 15% and between 0.2 and 18%. All these results confirm the efficacy of the present method for the determination of multi-residue pesticides in honey sample.
Pesticide levels in collected honey
The 18 collected samples were analyzed, according to the previously developed method, for the persistence of 84 pesticides. Residue levels were calculated with Xcalibur using calibration curves previously plotted.
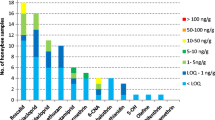
Most of the investigated samples have had some pesticides residues but of all the 84 pesticides sought 10 have been found in the majority of samples. The results are shown in Table 1.
The table above shows that, for the mentioned pesticides, the majority of samples contain residues with rates varying among regions.
Each apiary was studied for its contamination by 84 pesticide residues in honey matrixes. Pesticides contamination in honey usually results from bees’ contamination during foraging activities. In fact, bees have a high mobility and a wide flight area which makes them suitable for a wide monitoring area; honey bees fly up to 4 km in all directions from their apiary and thus have access to an area of about 50 km2 allowing them to monitor a large area and to detect different type of pollutants in their ambient environment (Malhat et al. 2015). Each of the studied apiaries was located in a rural area known for its agricultural productions. Thus, these sites are surrounded by cultivations what makes their emplacement somehow close to the site of phytosanitary products’ application.
These results show that collected samples from the Cedars of Lebanon were the less contaminated with pesticide residues. In fact, this region usually dedicated to organic crops, are the least contaminated with pesticides.
Contrariwise, Akkar valley seems to be the most contaminated area with pesticides residues. Fig. 2 shows a chromatogram corresponding to a sampling site in this region as well as the individual MRM traces for penconazole, Pyraclostrobine and Diflufenican given as an example for MRM fragmentation of pesticides residues. This chromatogram shows the concentration of the pollutants in this region. In fact, Akkar area is well known for its agricultural productions. We also note in this area the presence of organochlorine pesticides, considered as persistent pesticides. These pesticides are used in this zone with very weak control particularly at the Syrian borders where farmers are tempted to favor low cost and high efficiency on the back of ecological impacts (El-Osmani et al. 2014). In their work, El-Osmani et al. in 2014, showed the persistence of organochlorine pesticides in the ground water of Akkar district, confirming the use of these pesticides in this agricultural zone.
Furthermore, Byblos seems to be highly contaminated in hexachlorobenzene. This pesticide despite of its banning under the Stockholm convention on persistent organic pollutants on 2001 is still found at the environment (Specht et al. 2015). Hexachlorobenzene persist and bioaccumulate principally due to its high lipid solubility and resistance to degradation (Delisle et al. 2015).
For Batroun, the highest pesticides residues found belong to Diflufenican and Penconazol. The agricultural productions in this area explain the presence of these pesticides; in fact, this region is well known for its fruits and vegetables productions especially oranges, pome fruits and grapes. Penconazole is an agricultural fungicide which is used by foliar application to control a wide range of diseases in fruits and vegetables it is also applied to vegetables productions in order to control different mold and mildew which could explain the presence of this fungicide in abundance in this region.
Furthermore, Diflufenican is usually used for pre and post emergence of foliar absorbed herbicide for winter weed control in cereal crops which clarify its presence in Batroun also known for some cereal productions.
Several studies have shown the credibility of the use of honey as environmental bio indicator of pesticides contamination. In their study, Panseri et al. in 2014 showed a great direct relation between pesticides residues found in honey and contaminants sources (Panseri et al. 2014). Moreover, Malhat et al., in 2015 used honey samples as a gauge to monitor pesticides residues in ambient environment of the beehives in Egypt (Malhat et al. 2015). Furthermore, Italian organic honey was used by Chiesa et al. in 2016 in order to assess pesticides contamination in relation to environmental pollution (Chiesa et al. 2016).
Our results confirm the impact of intensive vegetables growing on honey contamination. It is important to underline that the systematic introduction of pesticides into nectar and pollen may have direct consequences for honey bee health and ultimately lead to pesticide contamination of honey and honey containing food (Panseri et al. 2014).
Conclusion
The developed method has enabled us to analyze 18 samples of honey originating from northern Lebanon. Indeed, to our knowledge, the combination of QuEChERS and SPME never was reported. Similarly, chromatographic methods coupled with mass spectrometry, offering high sensitivity, have enabled us quantification of pesticide residues even of very low dose less than 1 ng g−1.
In addition, the total concentrations of pesticides in honey recovered Akkar and Byblos were 1753.9 ng g−1 and 695.1 ng g−1 respectively. However, samples from the Cedars of Lebanon were the least concentrated pesticides with a total concentration of 90.2 ng g−1. This region is located in the mountain of Lebanon weakly cultivated dedicated to organic production.
The results of this study show that honey can reflect the specific pollution of a given environment and will be considered an appropriate sentinel for bio-environmental monitoring. This could be an effective tool for beekeepers to select production areas in particular for the production of organic honey.
References
Badiou-Bénéteau A, Benneveau A, Géret F, Delatte H, Becker N, Brunet JL, Reynaud B, Belzunces L (2013) Honeybee biomarkers as promising tools to monitor environmental quality. Environ Int 60:31–41
Barakat AA, Badawy HM, Salama E, Attallah E, Maatook G (2007) Simple and rapid method of analysis for determination of pesticide residues in honey using dispersive solid phase extraction and GC determination. J Food Agric Environ 5:97
Bargańska Ż, Ślebioda M, Namieśnik J (2013) Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control 31:196–201
Chiesa LM, Labella GF, Giorgi A, Panseri S, Pavlovic R, Bonacci S, Arioli F (2016) The occurrence of pesticides and persistent organic pollutants in Italian organic honeys from different productive areas in relation to potential environmental pollution. Chemosphere 154:482–490
Debayle D, Dessalces G, Grenier-Loustalot MF (2008) Multi-residue analysis of traces of pesticides and antibiotics in honey by HPLC-MS-MS. Anal Bioanal Chem 391:1011–1020
Delisle A, Ferraris E, Plante I (2015) Chronic exposure to hexachlorobenzene results in down-regulation of connexin43 in the breast. Environ Res 143:229–240
El-Osmani R, Net S, Dumoulin D, Baroudi M, Bakkour H, Ouddane B (2014) Solid phase extraction of organochlorine pesticides residues in groundwater (Akkar Plain, North Lebanon). Int J Environ Res 8:903–912
Gómez-Pérez ML, Plaza-Bolaños P, Romero-González R, Martínez-Vidal JL, Garrido-Frenich A (2012) Comprehensive qualitative and quantitative determination of pesticides and veterinary drugs in honey using liquid chromatography–Orbitrap high resolution mass spectrometry. J Chromatogr A 1248:130–138
Kujawski MW, Namieśnik J (2011) Levels of 13 multi-class pesticide residues in Polish honeys determined by LC-ESI-MS/MS. Food Control 22:914–919
Malhat FM, Haggag MN, Loutfy NM, Osman MA, Ahmed MT (2015) Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 120:457–461
Panseri S, Catalano A, Giorgi A, Arioli F, Procopio A, Britti D, Chiesa L (2014) Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control 38:150–156
Paradis D, Bérail G, Bonmatin JM, Belzunces LP (2014) Sensitive analytical methods for 22 relevant insecticides of 3 chemical families in honey by GC-MS/MS and LC-MS/MS. Anal Bioanal Chem 406:621–633
Rial-Otero R, Gaspar E, Moura I, Capelo J (2007) Chromatographic-based methods for pesticide determination in honey: an overview. Talanta 71:503–514
Specht IO, Bonde JPE, Toft G, Giwercman A, Spanò M, Bizzaro D, Manicardi GC, Jönsson BA, Robbins WA (2015) Environmental hexachlorobenzene exposure and human male reproductive function. Reprod Toxicol 58:8–14
Tette PAS, Guidi LR, de Abreu Glória MB, Fernandes C (2016) Pesticides in honey: a review on chromatographic analytical methods. Talanta 149:124–141
Wiest L, Buleté A, Giroud B, Fratta C, Amic S, Lambert O, Pouliquen H, Arnaudguilhem C (2011) Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J Chromatogr A 1218:5743–5756
Acknowledgements
We would like to thank the AZM & SAADE Association and the Lebanese University for funding the project, and the University of Strasbourg for the international mobility aid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
This paper was selected from the First International Symposium on Materials, Electrochemistry and Environment, September 2016, Tripoli (Lebanon). The evaluation process was monitored by Guest Editors Mishra Ajay (South Africa), Tarik Chafik (Morocco), Ahmad El Moll (Lebanon), Elke Fries (Germany), and Didier Hauchard (France).
Rights and permissions
About this article
Cite this article
Al Alam, J., Fajloun, Z., Chabni, A. et al. The use of honey as environmental biomonitor of pesticides contamination in northern Lebanon. Euro-Mediterr J Environ Integr 2, 23 (2017). https://doi.org/10.1007/s41207-017-0034-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-017-0034-9