Abstract
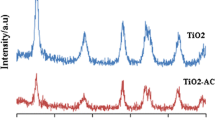
Photocatalysis is an alternative wastewater treatment method for the removal of toxic pollutants. The present work describes the hydrothermal synthesis of a new photocatalytic composite material involving activated carbon (PAC) and titanium dioxide nanoparticles (TiO2-NP) and its application for removal of Alizarin Red S (ARS) dye from wastewater. Characterization of the TiO2-NP/PAC composite was done by different analytical methods like SEM–EDX, XRD, FT-IR, photoluminescence, pHZPC and UV–visible diffuse reflectance spectroscopy. In order to evaluate the efficacy of the composite in the simultaneous adsorption and degradation of an anionic dye, ARS aqueous solution at varying TiO2-NP/PAC dosage, contact time, ARS concentrations and pH was evaluated. A composite dosage of 0.05 g shows 99.10% degradation of 20 mg l−1 ARS dye concentration in 80 min contact time at pH 2. It was observed that photocatalytic degradation of the dye followed pseudo-first-order rate kinetics and the adsorption isotherm was found to be best fitted with Freundlich isotherm model. The negative enthalpy and free energy indicated an exothermic and spontaneous process. DFT studies were used to explain the mechanism of formation of the composite, and results revealed a favourable immobilization of TiO2-NP on PAC via O-linkage of the O–Ti–O bond resulting in the formation of TiO2-NP/PAC composite. Chemical descriptors such as dipole moment, ionization energy, chemical softness and hardness and HOMO–LUMO energy obtained from DFT studies helped in understanding the comparative efficiency and reactivity of PAC and TiO2-NP/PAC towards ARS degradation.







Similar content being viewed by others
References
Vijayaraghavan K, Teo TT, Balasubramanian R, Joshi UM (2009) Application of Sargassum biomass to remove heavy metal ions from synthetic multi-metal solutions and urban storm water runoff. J Hazard Mater 164:1019–1023. https://doi.org/10.1016/j.jhazmat.2008.08.105
Zucca P, Vinci C, Sollai F et al (2008) Degradation of Alizarin Red S under mild experimental conditions by immobilized 5,10,15,20-tetrakis(4-sulfonatophenyl) porphine–Mn(III) as a biomimetic peroxidase-like catalyst. J Mol Catal A Chem 288:97–102. https://doi.org/10.1016/j.molcata.2008.04.001
Rais Ahmad AM (2013) Green synthesis of Xanthan gum/Methionine-bentonite nanocomposite for sequestering toxic anionic dye. Surf Interfaces 2:2–10. https://doi.org/10.1016/B978-0-08-096984-8.00029-X
Vandevivere PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J Chem Technol Biotechnol 72:289–302. https://doi.org/10.1002/(SICI)1097-4660(199808)72:4%3C289:AID-JCTB905%3E3.0.CO;2-%23
Petrovic M, Radjenovic J, Barcelo D (2011) Advanced oxidation processes (AOPs) applied for wastewater and drinking water treatment. Elimination of pharmaceuticals. Holist Approach Environ 1:63–74. https://hrcak.srce.hr/69233
Bahrudin NN, Nawi MA (2018) Immobilized titanium dioxide/powdered activated carbon system for the photocatalytic adsorptive removal of phenol. Korean J Chem Eng 35:1532–1541. https://doi.org/10.1007/s11814-018-0062-4
Ouzzine M, Romero-Anaya AJ, Lillo-Ródenas MA, Linares-Solano A (2014) Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon N Y 67:104–118. https://doi.org/10.1016/j.carbon.2013.09.069
Zhou W, Zhang P, Liu W (2011) Anatase TiO2 nanospindle/activated carbon (AC) composite photocatalysts with enhanced activity in removal of organic contaminant. Int J Photoenergy 2012:28–30. https://doi.org/10.1155/2012/325902
Anyika C, Asri NAM, Majid ZA et al (2017) Synthesis and characterization of magnetic activated carbon developed from palm kernel shells. Nanotechnol Environ Eng 2:1–25. https://doi.org/10.1007/s41204-017-0027-6
Matos J, Laine J, Herrmann JM (2001) Effect of the type of activated carbons on the photocatalytic degradation of aqueous organic pollutants by UV-irradiated titania. J Catal 200:10–20. https://doi.org/10.1006/jcat.2001.3191
Blantocas GQ, Alaboodi AS, Mekky A, Baset H (2018) Synthesis of chitosan–TiO2 antimicrobial composites via a 2-step process of electrospinning and plasma sputtering. Arab J Sci Eng 43:389–398. https://doi.org/10.1007/s13369-017-2695-8
de Souza TNV, de Carvalho SML, Vieira MGA et al (2018) Adsorption of basic dyes onto activated carbon: experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl Surf Sci 448:662–670. https://doi.org/10.1016/j.apsusc.2018.04.087
Ullah H, Tahir AA, Mallick TK (2016) Polypyrrole/TiO2 composites for the application of photocatalysis. Sens Actuators B Chem. https://doi.org/10.1016/j.snb.2016.10.019
Li W, Zeng T (2011) Preparation of TiO2 anatase nanocrystals by TiCl4 hydrolysis with additive H2SO4. PLoS ONE 6:2–7. https://doi.org/10.1371/journal.pone.0021082
Bhomick PC, Supong A, Baruah M et al (2018) Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: isotherm, kinetic, thermodynamic and regeneration studies. Sustain Chem Pharm 10:41–49. https://doi.org/10.1016/j.scp.2018.09.001
Asiltürk M, Şener Ş (2012) TiO2-activated carbon photocatalysts: preparation, characterization and photocatalytic activities. Chem Eng J 180:354–363. https://doi.org/10.1016/j.cej.2011.11.045
Faria PCC, Órfão JJM, Pereira MFR (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res 38:2043–2052. https://doi.org/10.1016/j.watres.2004.01.034
Belayachi H, Bestani B, Benderdouche N, Belhakem M (2015) The use of TiO2 immobilized into grape marc-based activated carbon for RB-5 Azo dye photocatalytic degradation. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.040
Frisch MJ, Trucks GW, Schlegel HB et al (1988) Gaussian 09, revision A. 02. Gaussian, Inc, Wallingford, CT, 2009
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys. https://doi.org/10.1063/1.447079
Bendjeddou A, Abbaz T, Gouasmia A, Villemin D (2016) Quantum chemical descriptors of some p-aminophenyl tetrathiafulvalenes through density functional theory (DFT). Acta Chim Pharm Indica 6:32–44
Cárdenas C (2011) The Fukui potential is a measure of the chemical hardness. Chem Phys Lett 513:127–129. https://doi.org/10.1016/j.cplett.2011.07.059
Bendjeddou A, Abbaz T, Gouasmia A, Villemin D (2016) Molecular structure, HOMO–LUMO, MEP and Fukui function analysis of some TTF-donor substituted molecules using DFT (B3LYP) calculations. Int Res J Pure Appl Chem 12:1–9. https://doi.org/10.9734/irjpac/2016/27066
Gázquez JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting powers. J Phys Chem A 111:1966–1970. https://doi.org/10.1021/jp065459f
Cortés-Arriagada D, Sanhueza L, Santander-Nelli M (2013) Modeling the physisorption of bisphenol A on graphene and graphene oxide. J Mol Model 19:3569–3580. https://doi.org/10.1007/s00894-013-1872-2
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9:7168–7175. https://doi.org/10.1039/c1ob05856h
Pratihar S, Roy S (2010) Nucleophilicity and site selectivity of commonly used arenes and heteroarenes. J Org Chem 75:4957–4963. https://doi.org/10.1021/jo100425a
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J Mol Struct Theochem 865:68–72. https://doi.org/10.1016/j.theochem.2008.06.022
Singh P, Vishnu MC, Sharma KK et al (2016) Photocatalytic degradation of Acid Red dye stuff in the presence of activated carbon–TiO2 composite and its kinetic enumeration. J Water Process Eng 12:20–31. https://doi.org/10.1016/j.jwpe.2016.04.007
Zhao Y, Li B, Wang Q et al (2014) NiTi-Layered double hydroxides nanosheets as efficient photocatalysts for oxygen evolution from water using visible light. Chem Sci 5:951–958. https://doi.org/10.1039/c3sc52546e
Mohapatra L, Parida K (2016) A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J Mater Chem A 4:10744–10766. https://doi.org/10.1039/c6ta01668e
Zhang N, Yang MQ, Liu S et al (2015) Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem Rev 115:10307–10377. https://doi.org/10.1021/acs.chemrev.5b00267
Chowdhury PR, Bhattacharyya KG (2016) Synthesis and characterization of Mn/Co/Ti LDH and its utilization as a photocatalyst in visible light assisted degradation of aqueous Rhodamine B. RSC Adv 6:112016–112034. https://doi.org/10.1039/c6ra24288j
Zheng P, Pan Z, Li H et al (2015) Effect of different type of scavengers on the photocatalytic removal of copper and cyanide in the presence of TiO2@yeast hybrids. J Mater Sci Mater Electron 26:6399–6410. https://doi.org/10.1007/s10854-015-3229-3
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Anyika C, Asri NAM, Majid ZA et al (2017) Batch sorption–desorption of As (III) from waste water by magnetic palm kernel shell activated carbon using optimized Box–Behnken design. Appl Water Sci 7:4573–4591. https://doi.org/10.1007/s13201-017-0610-9
Frendulich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Ahmad R, Mirza A (2018) Synthesis of Guar gum/bentonite a novel bionanocomposite: isotherms, kinetics and thermodynamic studies for the removal of Pb(II) and crystal violet dye. J Mol Liq 249:805–814. https://doi.org/10.1016/j.molliq.2017.11.082
Ahmad R (2009) Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater 171:767–773. https://doi.org/10.1016/j.jhazmat.2009.06.060
Ahmad R, Mirza A (2018) Adsorptive removal of heavy metals and anionic dye from aqueous solution using novel Xanthan gum-Glutathione/Zeolite bionanocomposite. Groundw Sustain Dev 7:305–312. https://doi.org/10.1016/j.gsd.2018.07.002
Atout H, Bouguettoucha A, Chebli D et al (2017) Integration of adsorption and photocatalytic degradation of methylene blue using TiO2 supported on granular activated carbon. Arab J Sci Eng 42:1475–1486. https://doi.org/10.1007/s13369-016-2369-y
Xing B, Shi C, Zhang C et al (2016) Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation. J Nanomater. https://doi.org/10.1155/2016/8393648
Subramani AK, Byrappa K, Ananda S et al (2007) Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull Mater Sci 30:37–41. https://doi.org/10.1007/s12034-007-0007-8
Zhong HE, Shaogui YANG, Yongming JU, Sun C (2009) Microwave photocatalytic degradation of Rhodamine B using TiO2 supported on activated carbon: mechanism implication. J Environ Sci 21:268–272
Le HA, Linh LT, Chin S, Jurng J (2012) Photocatalytic degradation of methylene blue by a combination of TiO2-anatase and coconut shell activated carbon. Powder Technol 225:167–175. https://doi.org/10.1016/j.powtec.2012.04.004
Andriantsiferana C, Mohamed EF, Delmas H (2014) Photocatalytic degradation of an azo-dye on TiO2/activated carbon composite material. Environ Technol (United Kingdom) 35:355–363. https://doi.org/10.1080/09593330.2013.828094
Acknowledgements
Mridushmita Baruah is grateful to UGC Non-NET fellowship, and Aola Supong, Parimal Chandra Bhomick and Rituparna Karmaker are grateful to the DST-INSPIRE Fellowship for financial assistance. Support under DST-FIST is acknowledged. Funding was supported by the UGC Non-NET Fellowship (Grant No. PF/RDC/NNF-41/2017-1521 dated 31/05/2017) and Department of Science and Technology, New Delhi (IN), INSPIRE Fellowship (Grant Nos., IF160718, IF150297 and IF160719). The authors are also grateful to Nikhil Guchhait, Department of Chemistry, Calcutta University, for giving computational facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baruah, M., Supong, A., Bhomick, P.C. et al. Batch sorption–photodegradation of Alizarin Red S using synthesized TiO2/activated carbon nanocomposite: an experimental study and computer modelling. Nanotechnol. Environ. Eng. 5, 8 (2020). https://doi.org/10.1007/s41204-020-00071-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-020-00071-3