Abstract
Selective catalytic reduction (SCR) systems are the state-of-the-art technology to reduce nitrogen oxide emissions (NOx) of modern diesel engines. The system behaviour is well understood in the common temperature working area. However, the system properties below light-off temperature are less well known and offer a wide scope for further investigations. Vehicle measurements show that under specific conditions during cold start, NOx can be partially stored and converted on on-filter and flow-through SCR catalysts. The purpose of this work was in a first step to analyse the main influence parameters on the NOx storage behaviour. Therefore, synthetic gas test bench measurements have been carried out, varying the gas concentrations, temperature, and gas hourly space velocity (GHSV). These investigations showed that the NOx storage effect strongly depends on the NH3 level stored in the catalyst, GHSV, the adsorbed water (H2O) on the catalyst, and the temperature of the catalyst. Further influence parameters such as the gas composition with focus on carbon monoxide (CO), short-chain hydrocarbons and long-chain hydrocarbons have been analysed on a synthetic gas test bench. Depending on operating conditions, a significant amount of NOx can be stored on a dry catalyst during the cold start phase. The water vapor from the combustion condenses on the cold exhaust pipe during the first seconds, or up to a few minutes after a cold start. As the water vapor reaches the surface of the catalyst, it condenses and adsorbs onto it, leading to a sudden temperature rise. This exothermal reaction causes the stored NOx to be desorbed, and furthermore it is partially reduced by the NH3 stored in the catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Even though the market share of electrically powered passenger cars has increased in the European market over the last few years, the share of diesel engines in new passenger cars was still 30.5% in the year 2018. Petrol-driven cars showed even a higher share for new registered cars in the European Union in the year 2018 of almost 60%. [1]
The fact that a diesel engine has lower CO2 emissions than a petrol-powered passenger car, resulting from its significant advantage in thermodynamic efficiency, underpins its importance to reach overall CO2 fleet emission targets [2].
One of the main development targets for future diesel vehicles is to fulfil new emissions regulations such as Euro 7 including real driving emissions legislations (RDE). Main targets are NOx and particulate matter emissions, causing a need for a complex exhaust aftertreatment system for modern diesel vehicles. These systems typically consist of a diesel oxidation catalyst (DOC), a diesel particulate filter (DPF) with SCR coating, a selective catalytic reduction catalyst (SCR), and eventually an NH3 slip catalyst (ASC) [3,4,5].
The DPF with SCR coating has major advantages compared to two separate systems, such as a better packaging, as well as an improved heating up of both subsystems due to the possible closed-couple position. These facts lead to an improved cold start emission performance. Furthermore the use of a DPF with SCR coating and a normal SCR catalyst offers a greater overall functional SCR volume, which is essential to assure high NOx reduction rates at high exhaust mass flows, respectively high gas hourly space velocities (GHSV). The SCR on filter catalyst is a state-of-the-art technology for NOx and particulate matter emission reduction in passenger vehicles [6,7,8].
The main design criterion of a DPF with SCR coating is a good urea mixture preparation in the exhaust system upstream of the catalyst. In addition to that requirement, the chosen temperature to start the urea dosing is also a key performance indicator. In general urea depositions are not relevant if those two criteria are met. Moreover the clogging of the filter pores by means of solid urea decompositions are in general, neglectable. Further studies show that for lower temperatures a soot loaded catalyst shows a lower NOx conversion rate compared to a soot free catalyst [9, 10].
A second thermally decoupled underfloor SCR catalyst offers a further increase of the NOx reduction potential. This is particularly the case during high temperature phases, such as a particulate-filter regeneration and high load operation. A double dosing aftertreatment system with two separate SCR catalysts enables a more efficient use of urea, thus causing a lower urea consumption. The double dosing systems allows an independent operation of the close-coupled SCR and underfloor SCR systems [8].
The carbon-monoxide and hydrocarbon emissions of a diesel vehicle can be reduced by a diesel oxidation catalyst. A three-way catalyst cannot be used to tackle the NOx emissions due to the lean combustion in a diesel engine. Well over a decade now, the diesel particulate filter has been the state-of-the-art technology to reduce particulate matter emissions. Up to now one of the major development challenges has been to meet future NOx limitations. Currently a close-coupled SCR system combined with an underfloor SCR system is used. Furthermore, depending on the vehicle size either one or two urea dosing systems are used [11].
Over the past years, each of these components has been developed further with respect to system performance and overall understanding of the reaction mechanisms by several research groups. In the SCR itself a lot of different reactions can take place, the most regarded reaction path is the so called “standard” SCR reaction (\(4 NO+4 N{H}_{3}+{O}_{2}\to 4 {N}_{2}+6{ H}_{2}O\)) [12,13,14]. The presence of NO2 in a ratio of 1:1 for NO:NO2 enables the “fast” SCR reaction (\(NO+N{O}_{2}+2 N{H}_{3}\to 2 {N}_{2}+3 {H}_{2}O\)), which is quicker than the “standard” SCR reaction and starts at lower temperatures. Another reaction is the so called “NO2” SCR reaction (\(6 N{O}_{2}+8 N{H}_{3}\to 7 {N}_{2}+12 {H}_{2}O\)), which has two significant drawbacks: on the one hand the “NO2” SCR reaction requires a higher amount of ammonia, and the reaction itself is also slower [12,13,14,15,16,17,18].
The SCR system behaviour as well as the underlying reactions are well understood and can be predicted with adequate models in the common operating temperature range from approx. 175 °C up to 650 °C [15, 19]. For the system evaluation, catalyst temperatures below 175 °C are often not considered, because SCR reactions play a minor part in the NOx reduction in this temperature area. At temperatures below 175 °C NH3 is only available as stored NH3 in the catalyst itself, due to the fact that the urea dosing needs a certain temperature level to ensure complete thermolysis reaction (\({\left({NH}_{2}\right)}_{2}CO\to {NH}_{3}+HNCO\)) and the following hydrolysis reaction (\(HNCO+{H}_{2}O\to {NH}_{3}+{CO}_{2}\)) to create NH3. However, SCR catalysts can store NH3 up to a certain amount, depending on its zeolite structure and chemical composition [19,20,21,22,23,24].
Current studies have investigated the NOx storage behaviour and point out the negative effect of water. Water inhibits the oxidation of NO and has a negative effect on the NOx storage itself. Beyond that even a small amount of water is detrimental on the NOx storage behaviour. The presence of water during the adsorption causes a lower peak in temperature during the NOx desorption phase, leading to a lower NOx reduction potential. Furthermore these papers aim to enhance the NOx storage effect by improving the storage material, as well as getting a better understanding of possible influence parameters [4, 25, 26].
Several reasons such as the lower exhaust gas temperatures due to higher engine efficiency and more stringent emissions regulations move the field of interest of the SCR system behaviour to lower catalyst temperatures (< 175 °C). Vehicle measurements for a commercial Cu-zeolite SCR catalyst show that during cold start a variety of effects interact and influence the SCR system behaviour. The aim of this work is on the one hand to get a better understanding of the interactions and the effect mechanisms itself, and on the other hand to create a basis to be able to design a model which predicts the system behaviour at these temperatures. Lab scale tests with a synthetic gas test bench have been carried out with the aim to understand the main influence parameters on the adsorption and desorption processes of the NOx emissions on the SCR catalyst. Beyond this, NOx reduction in the low temperature range has been analysed with the main focus on the gas composition as an influence parameter.
2 Vehicle measurements
2.1 Methods
During cold start vehicle measurements, a NOx storage effect as well as a water adsorption effect were observed. These measurements indicate that there is a coherence between the NOx storage effect and the water adsorption effect. In order to validate the NOx storage behaviour and detect further influence parameters, vehicle measurements were carried out with the same catalyst technology, parallel to the lab scale tests on the synthetic gas test bench (Fig. 4). The test vehicle was equipped with a 3.0 l common-rail diesel engine, a close-coupled SCR system and an underfloor SCR system. The gas sampling points as well as the additional temperature sensors can be seen in Fig. 1. At the gas sampling ports, the gas phase was analysed with additional commercially available NOx and NH3 sensors, whereby the NOx sensors were already active at the start of the vehicle tests.
For the vehicle measurements a conditioning cycle was carried out, which enhanced the repeatability for each measurement. After a soak time of at least 8 h to guarantee catalyst temperatures below 50 °C, the actual measurement cycle was driven. The measurement cycle represents a city cycle, which is followed by extra-urban driving that acts as the conditioning cycle for the next measurement, with a total distance of 42 km. The temperature profile and the vehicle speed are shown in Fig. 2. The entire vehicle measurements were carried out on public roads.
3 Results and discussion
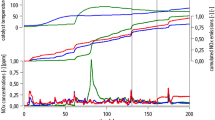
The measurement cycle described above was carried out with focus on the first few hundred seconds of the cycle only. The reason for this is that the NOx adsorption and desorption cycle is completed after the first few hundred seconds. The NOx concentrations engine out, after the close-coupled SCR system, and downstream of the underfloor SCR system for the first 300 s after a cold start are depicted in Fig. 3. For the first 75 s all the NOx emissions stay absence after the close-coupled SCR system. After 75 s engine out NOx emissions are well below 100 ppm, at about 95 s a NOx emission peak occurs after the close-coupled SCR system. This sharp NOx-emissions increase is caused by the temperature rise due to water adsorption in the catalyst, as it can be seen in various literature [4, 25, 27].
Measurement data for the first 300 s during a cold start of the measurement cycle in accordance to the measurement setup in Fig. 1
Furthermore, the NOx emissions after the underfloor SCR system stay zero for the first 130 s. After 130 s a slow increase of NOx emissions downstream of the underfloor SCR system can be noticed, which results in a sharp NOx emissions peak at 170 s. The slow increase of the NOx emissions can be explained through NOx slip caused by a peak in the exhaust mass flow. The following sharp NOx-emissions peak is caused by the temperature rise.
Beyond 200 s the NOx emissions of both SCR catalyst sampling points are nearly the same, however, the engine out NOx emissions values are slightly higher. In general, the behaviour of the close-coupled and the underfloor SCR systems are the same, but the effect is pronounced stronger for the latter. There could be several reasons for this difference such as volumetric varieties, substrate types, or the surface-to-volume ratio of the catalysts. The exhaust gas during the NOx peaks downstream of both SCR systems consists entirely of NO only.
The cold start temperature behaviour for the entire vehicle setup with its additional thermocouple elements can be seen in Fig. 3. The engine out exhaust-gas temperature increases continuously as it would be expected for a heating-up process of the exhaust system. The temperature measurements in the catalysts show a rather untypical temperature profile for the first few hundred seconds, the behaviour is similar to that of the NOx emissions for each catalyst. The trend of the temperature profile as well as the NOx-emissions profile corresponds to already existing literature, however, absolute values cannot be compared due to a lack of data concerning the catalyst composition and volume [4]. For the first 80 s the temperature in the close-coupled SCR system (blue line) stagnates at ca. 20 °C. Between 80 and 115 s a sudden increase in temperature of more than 100 K occurs. After the sudden temperature rise the temperature decreases till it reaches 90 °C, before slowly increasing again at 275 s.
The temperature profiles (for the thermocouples) in the underfloor SCR system follow the same principle as for the close-coupled SCR system. With the two thermocouples an unequal distribution in the temperature rise for the inlet and the outlet area can be determined. The lift in temperature is nearly double the amount (130 K) in the outlet section of the catalyst than compared to the inlet area (68 K). The underfloor catalyst reaches a maximum temperature during the first 300 s of 156 °C in the depicted measurement cycle. As already shown for the NOx peak, the temperature profile in the underfloor SCR system behaves in a much more pronounced way than in the close-coupled SCR system. Between 300 and 600 s the outlet temperature decreases and subsequently rises with the other measured temperatures.
The temperature behaviour is caused by the water in the exhaust gas. Whereby the behaviour over the SCR catalysts must be an exothermic H2O-adsorption reaction, followed by an endothermic H2O-desorption. The temperature rise is due to H2O-adsorption on the small pores of the zeolite. Afterwards an endothermic H2O-desorption occurs, caused by the evaporation of the condensed water. The entire temperature behaviour can be seen in Fig. 2. This effect can be found similarly during water dosing over different zeolite types in other literature [27, 28].
There are two main sources of water in the exhaust gas, on the one hand water contained in the intake air and on the other hand water from the combustion itself. Typical water content in the exhaust gas is between 6 and 12% by volume. During cold start, water in the exhaust gas can condense on the cold components of the exhaust manifold, as well as on the piping, DOC and so on. This condensation process continues as long as the exhaust pipes and monoliths are below the condensation temperature. The condensed water reduces the moisture content in the exhaust gas, enabling the delayed temperature increase in the SCR systems. As the exhaust components upstream of the close-coupled SCR system are saturated with water, the moist exhaust gas reaches the surface of the SCR catalyst. Furthermore, the water content in the exhaust increases due to the heating up of the upstream exhaust parts by evaporating the previously condensed water. Due to the high surface area and the cold temperature of the SCR catalyst, a high amount of water can condense and secondly adsorb on the catalyst, causing the sharp temperature increase in the catalyst. The higher rise in temperature at the outlet area of the underfloor SCR system compared to the inlet section can be explained by the fact that the exhaust gas that reaches the rear part of the catalyst is already preheated by the inlet area, and the increased humidity in the exhaust gas due the evaporation in the upstream components.
In regard to the NOx emissions behaviour an adsorption and desorption process describes the activities best. Moreover, only a certain amount of the adsorbed NOx desorbs during the temperature rise, leaving a NOx reduction. A possible way to explain the NOx reduction is that the local temperature, during water adsorption is much higher than the measured one. In combination with NH3 adsorbed on the surface of the catalyst enables a functional NOx reduction process. These topics are investigated in the synthetic gas test bench measurements. Additionally, a time-dependent correlation between the temperature rise and the NOx desorption peaks is found.
4 Synthetic gas test bench measurements
4.1 Methods
To analyse the results of vehicle measurements, more precisely a detailed study of several parameters was done on a synthetic gas test bench. Methods and Results are discussed in the following section.
4.2 Catalyst samples
For the synthetic gas test bench catalyst samples with a diameter of 1 inch were core drilled out of a conventional SCR catalyst. To achieve higher space velocities the bore sample was cut to a length of 3 inch. The SCR catalysts analysed in this work are monolithic Cu-zeolites with a cell density of 400 cells per square inch.
4.3 Synthetic gas test bench
To determine the main influence parameters on the adsorption, desorption, and NOx reduction behaviour the usage of a synthetic gas test bench is the first choice, due to its high repeat accuracy and the possibility to adapt each variation parameter independently from each other. The layout of the synthetic gas test bench with its subcomponents and gas compositions can be seen in Fig. 4. The synthetic gas test bench uses mass flow controllers to compile the individual gas mix. Water and C10H22 are the only two components which are dosed as a liquid in combination with an evaporator device, the other components are added gaseous. Nitrogen acts as a carrier gas to define the needed gas flow.
For temperature regulation a PID controller is used, which defines how much gas must be heated by the gas heater, the rest is bypassed to the mixing unit. The gas temperature sensor upstream of the catalyst sample acts as an input variable for the temperature regulation. To evaluate the temperature behaviour over the catalysts length, two additional thermocouple elements were placed in the sample, which can be seen in Fig. 5. The reactor itself is made from quartz glass. The catalyst sample is placed in a ceramic sample holder, which is attached to a stainless-steel mounting unit. The synthetic gas is analysed after the catalyst sample by means of a Fourier-transform infrared spectroscopy (FTIR), a paramagnetic analyser (PMA) and a nondispersive infrared sensor (NDIR). The FTIR is used to analyse NOx, NH3, H2O and so on. The NDIR measures the CO and CO2 content in the synthetic gas and the PMA determines the oxygen content.
Before each test a conditioning procedure was carried out by heating the sample up to 450 °C in a gas mixture of 8% O2, 8% CO2 in N2 as a filler gas at a space velocity of 25,000 [1/h]. The temperature of 450 °C was kept for at least 500 s, to guarantee that the piping and the catalyst sample were free of water. The gas concentration range as well as the dosed components fed to the reactor are depicted in Table 1.
The low temperature range below 150 °C was the main scope of investigation, which is typical during a cold start of an engine. However measurements started at 50 °C for the reactor setup to secure a proper temperature regulation. The NOx storage effect was also evaluated for higher starting temperatures such as 75, 100 and 150 °C. The piping up- and downstream of the reactor were heated to 115 °C in order to avoid water condensation, therefore only in the quartz glass tube water condensation could occur. The space velocity was varied between 15,000 and 50,000 [1/h], whereas the very low and high space velocity was only selected to analyse the influence of space velocity. The test setups carried out in this paper to evaluate the different influence parameters are shown in Table 2. Furthermore a test series was conducted to determine the water adsorption and desorption behaviour. The test procedure is shown in Fig. 6 and consisted of an optional preloading step, during which NH3 and/or water was loaded on the catalyst. In the adsorption step the GHSV, the catalyst temperature, gas temperature, as well as the gas composition were varied.
5 Results and discussion
5.1 Adsorption/desorption behaviour of water
The H2O adsorption/desorption test setup raises several questions like: is there an influence of the water concentration on the adsorption behaviour, or what is the maximum temperature rise under the given boundary conditions of the synthetic gas test bench in a lab scale format. For the given test setup, the lowest possible test starting temperature is 50 °C, at a space velocity of 25,000 1/h. The gas composition for the adsorption/desorption tests can be seen in Table 3. The preconditioning guarantees a water free piping and test sample, the total time with a temperature above 100 °C is over 55 min. Before the adsorption phase begins the starting temperature is adjusted to 50 °C. During the adsorption phase 10% water is dosed for 70 s. After this soak period the water dosage is shut off again and the sample is flushed with dry synthetic gas until the measured water content after the sample drops below 500 ppm. This step removes the loosely bound water from the quartz glass reactor and the catalyst sample. After this debouncing phase a temperature programmed desorption (TPD) is started with an increase of 25 K/min up to 450 °C.
The synthetic gas test bench measurement (Fig. 7) confirms the theory that the adsorption of water leads to a distinctive exothermic behaviour, a temperature rise of 121 K can be detected. Additionally, the endothermic performance can be seen as the water dosing is shut off during the debouncing as well as desorption phases. The desorption of the loosely bound water only leads to a temperature drop of 12 K downstream of the catalyst sample, whereas during the TPD a maximum temperature difference of 77 K can be determined. Overall the temperature profile after the catalyst sample is in accordance with vehicle measurements. Additional measurements show that the temperature rise significantly decreases to 50 °K for a water dosing of 2%, displaying that the water concentration has a major influence on the exothermic behaviour.
5.2 Principal adsorption/desorption behaviour of NOx
To validate the NOx adsorption and desorption behaviour a test setup similar to the water adsorption/desorption test has been established. The test setup (Table 4) consists of a preconditioning phase, followed by a phase of constant temperature where 50 ppm of NO is dosed until a NOx break-through of 45 ppm is measured. The starting temperature of 50 °C for the NOx storage test is the same as for the water storage test, as well as the space velocity of 25,000 [1/h] and the entire preconditioning phase.
During the adsorption phase only NO is dosed due to the fact that the NOx composition during cold start vehicle measurements also show only NO upstream of the catalyst. The entire test is carried out without water dosing to replicate the adsorption phase of the vehicle measurements, where a lack of water is observed. As can be seen in Fig. 8, the NOx concentration downstream of the catalyst slowly increases, while NOx concentrations upstream of the catalyst are constant. After a NOx breakthrough of 45 ppm is reached, the NO dosage is shut off and the TPD begins. During the adsorption and the desorption phase a mass balancing of the dosed and measured NOx concentrations is carried out to validate the storage potential of the catalyst sample. During the desorption phase, two major NOx desorption peaks occur, the first peak reaches its maximum at an inlet temperature of 164 °C and the NOx composition is mainly NO. The second NOx desorption peak reaches its maximum at an inlet temperature of nearly 400 °C and primarily consists of NO2. The presence of these two desorption peaks leads to the assumption, that more than one storage site is available on the SCR catalyst.
The observation of two storage sites could not be validated by current literature [26], furthermore the desorption temperature of the stored NOx differs from the temperature range compared to other papers [4]. A possible explanation for this diverse behaviour is that different catalyst compositions have been used, suggesting that the desorption temperature also strongly depends on the catalyst coating.
Furthermore, the temperature driven desorption underpins the thesis that the adsorbed NOx desorbs by the rise in temperature caused by adsorption of water rather than competing storage sites. These findings are consistent with other literature [4, 24].
To evaluate the maximum storable amounts of NOx emissions for this technology the dosed NO has been integrated \((1\)) during the adsorption phase as well as the desorbed NOx during the TPD (2).
For the measurement seen in Fig. 8, 3.043 g/l NOx can be stored on the catalyst sample, the desorbed amount is with 3.281 g/l a little bit higher, this difference can be explained by measurement noise due to the longer adsorption phase. Such high NOx storage capacity could not be noticed in vehicle measurements. One possible reason is that during the vehicle measurements the exothermic water adsorption occurs and therefore stops the NOx adsorption earlier compared to the synthetic gas test bench measurements.
To evaluate the lower storage capacity of NOx during vehicle measurements, a test setup with a lower slip limit of 10 ppm NOx instead of 45 ppm during the adsorption phase has been carried out. The results for this test setup are shown in Fig. 9. Remarkable for this measurement is that for the lower NOx slip limit of 10 ppm, only the low temperature desorption peak is present. This leads to the assumption that the storage site with the lower desorption temperature and mainly consisting of NO is addressed first. This behaviour corresponds to the vehicle measurements, where the desorption peak also mainly consists of NO and the temperature range of the desorption is similar. Furthermore, the amount of stored NOx is significantly lower (0.265 g/l), but still considerable higher compared to vehicle data. In contrast to the measurement seen in Fig. 8 the adsorption phase only takes 13.5 min for the lower NOx slip limit instead of 106 min.
5.3 Parameter influence study for NOx storage behaviour
The measurements carried out to this point aimed to get a principal understanding of the NOx adsorption and desorption mechanisms, as well as the thermal behaviour of the catalyst caused by water adsorption and condensation. To get a wider insight of possible influence factors a parameter study has been carried out, therefore the test setup presented in Fig. 8 has been further adapted depending on the influence parameter. If not stated otherwise the gas composition for the following tests is 8% O2, 8% CO2 in N2 as a filling gas, at a space velocity of 25,000 1/h and an adsorption temperature of 50 °C.
5.4 Influence of the substrate type on NOx storage behaviour
To validate the impact of different substrate types on the NOx storage behaviour, the same test setup as shown in Fig. 8 for the flow-through SCR catalyst was carried out with an on-filter SCR catalyst, the result is visualized in Fig. 10.
The total amount of adsorbed NOx reaches a value of 0.835 g/l, which is significantly lower compared to the flow-through substrate. Furthermore, the first desorption peak reaches its maximum at a temperature of 200 °C and the second one is reached at 385 °C. The general NOx storage behaviour is comparable to the flow-through substrate, a possible reason for the lower storable NOx could be the lower amount of washcoat loading of the on-filter catalyst. The main design criterion for the close-coupled SCR system and the underfloor SCR system has already been mentioned in the introduction section, it is noteworthy that the NOx storage is up to now not a major design criterion for exhaust system development. Despite the fact of the lower NOx storage capacity of the SCR-on-filter catalyst, both catalyst types combined offer a greater NOx storage potential than a stand-alone version. Because the absolute amount of storable NOx scales with the total amount of washcoat available in the entire exhaust system, therefore a greater NOx reduction potential during the cold start is possible. The reason for the lower washcoat loading of the SCR-on-filter catalyst is that there is a trade-off between backpressure and washcoat loading.
5.5 Influence of the adsorption temperature on NOx storage behaviour
For the next variation parameter, the temperature during adsorption in the catalyst was varied to evaluate the sensitivity towards different cooling phases for vehicle application. The results for the temperature variation are visualized in Fig. 11. The results show that the NOx storage has a strong dependency on the catalyst temperature, this confirms the observations that the NOx adsorption occurs preferably at lower temperatures. The TPD (not illustrated) shows that both NOx desorption peaks are affected equally with increasing adsorption temperature.
5.6 Influence of the space velocity on NOx storage behaviour
Another influence parameter which has been detected in vehicle measurements is the space velocity in the catalyst. To tackle the severity of this variable, synthetic gas test bench measurements with different space velocities were carried out. As can be seen in Fig. 12, the space velocity plays a minor role with regard to the maximum NOx adsorption potential. However, doubling the space velocity from 25,000 to 50,000 1/h leads to a significant reduction of the time needed to reach a NOx slip of 10 ppm during the adsorption phase by more than 75%. This indicates that a higher space velocity leads to a disproportionate earlier NOx slip. The underlying cause of this phenomenon could potentially be that the time needed for a NOx molecule to adsorb on the catalyst increases as the saturation point of the possible storable amount of NOx is reached. Thus, a higher space velocity makes it impossible for some NOx molecules to adsorb on the catalyst and reduces the maximum storable NOx emissions for the same NOx slip limit. Moreover, the same test setup has been carried out with a space velocity of 15,000 1/h, this measurement points out that the time needed to cross the 10 ppm NOx slip point does not change compared to the setup with a space velocity of 25,000 1/h. This indicates that a certain minimum time is required for the NOx molecules for the adsorption process.
5.7 Influence of the exhaust gas composition on NOx storage behaviour
To evaluate the influence of other typical exhaust gas components during cold start such as short-chain hydrocarbons, long-chain hydrocarbons, and carbon monoxide, further lab scale measurements were carried out. Therefore, optionally C3H6, C10H22 and CO are added to the inlet gas composition during the NOx adsorption phase and shut off again during the desorption phase.
In Fig. 13 the NOx storage behaviour in combination with 2000 ppm of CO dosing is shown. A total amount of 1.714 g/l NOx can be stored, whereby CO dosing primarily affects the storage site with the higher NOx release temperature. Furthermore, both desorption peaks mainly consist of NO, which implies that the CO inhibits the oxidation of NO to NO2. The calculated mass balance reveals that a significant amount of the dosed CO oxidises to CO2. This reduces the available oxygen potentially causing the reduced oxidation of NO to NO2 during the adsorption phase, also leading to a reduced storage amount of NOx. Additionally, a test with 5000 ppm CO dosing was carried out, with similar results. Furthermore, the calculated mass amount of NOx during adsorption and desorption was the same for both tests.
In the next step, the influence of short-chain hydrocarbons was evaluated. For that purpose C3H6 was added in various concentrations during the NOx adsorption phase. The measurement results for 50 ppm C3H6 dosing are illustrated in Fig. 14. Like for the CO tests, the NOx desorption peaks mainly consist of NO, furthermore the second desorption peak is significantly lower than the first one.
It is notable that the C3H6 dosing slightly reduces the amount of stored NOx to 2.854 g/l and cuts the desorbed NOx to less than one third of the adsorbed NOx amount to 0.927 g/l. The adsorbed C3H6 on catalyst enables the reduction of stored NOx during the TPD. The low temperature NOx reduction is due to the following reaction \((3)\) [29, 30].
In Fig. 15 the NOx mass balance during adsorption and desorption for different C3H6 dosing concentrations are shown. The difference in the mass balance for the first case without C3H6 dosing is traced back to measurement inaccuracies due to the long duration of the adsorption phase. Overall for low C3H6 concentrations the amount of adsorbed NOx is not significantly influenced, though C3H6 enhances the reduction of the adsorbed NOx considerable. The reduced storability of NOx with higher C3H6 concentrations can be caused by a blocking effect of the NOx storage sites by the C3H6 molecules.
The same measurement procedure was carried out with C10H22 instead of C3H6. For the 250 ppm C10H22 dosing measurement, a total NOx amount of 0.853 g/l can be stored on the catalyst, whereby just 0.616 g/l of NOx desorb during the TPD. An explanation for this sharply reduced storage capacity is the larger molecule size of C10H22 compared to C3H6 and thus blocking the storage sites of NOx more easily. The reduction takes the formula \((4)\) as a basis [29, 30].
5.8 Influence of NH3 on NOx storage behaviour
A test series with focus on the influence of NH3 preloading on the catalyst sample was executed. The preloading of NH3 was achieved by adding an additional phase step before the NOx adsorption phase. A time-dependent NH3 dosing with a gas composition of 100 ppm NH3, 5% H2O, 8% CO2 and 8% O2 in N2 at 200 °C and a space velocity of 25,000 1/h was chosen to guarantee a valid mass balance for the NH3 storage level. As an example a preloading of 0.2 g/l NH3 is shown in Fig. 16, the space velocity and temperature were chosen in a way so that no NH3 slip occurs. The initial overshoot of the NH3 concentration at the start of the NH3 dosing is caused by the mass flow controller.
The addition of water during the NH3 dosing prevents the deposition of NH3 on the piping of the synthetic gas test bench. The NOx storage results for different NH3 loadings are visualized in Fig. 17. As soon as NH3 is stored on the catalyst, the NOx storage capacity drops from 3.043 g/l to about 1.4 g/l. It is noteworthy that for a NH3 storage level between 0.2 and 0.5 g/l the amount of stored NH3 reveals no significant influence on the NOx storage capacity. During the TPD a NOx reduction of 0–100 mg/l occurs, whereby the maximum NOx reduction is reached at a NH3 storage level of 0.5 g/l. This behaviour corresponds to the general understanding from the vehicle measurements that a higher NH3 storage level leads to a higher NOx reduction potential during cold start. During the NOx desorption phase and simultaneous NOx reduction phase not all the stored NH3 is used for NOx reduction, the remaining NH3 is available on the catalyst. For example if 100 mg of NOx is converted, a total amount of 57 mg of NH3 is consumed by the reaction. This corresponds to a NH3 storage level reduction of about 11.5% at an NH3 storage level of 0.5 g/l. It is assumed that the NOx reduction takes predominantly the standard SCR reaction path (\(4 NO+4 N{H}_{3}+{O}_{2}\to 4 {N}_{2}+6{ H}_{2}O\)) and partially the fast SCR reaction path (\(NO+N{O}_{2}+2 N{H}_{3}\to 2 {N}_{2}+3 {H}_{2}O\)) [12,13,14]. This is the reason for the results of the lower NOx slip limit test, where only one desorption peak occurs, which mainly consists of NO. That behaviour is similar to vehicle measurements.
5.9 Influence of adsorbed H2O on NOx storage behaviour
The last topic of the synthetic gas test bench experiments was to evaluate the influence of water adsorption on the NOx storage behaviour. Vehicle measurements showed a strong inhibition effect due to stored water on the NOx storage activities. A similar preloading phase like that for the NH3 synthetic gas test bench measurements described above was introduced. The water preloading phase utilizes a time dependent H2O dosing of 2% H2O, 8% CO2 and 8% O2 in N2 at 100 °C and a space velocity of 25,000 1/h. The general behaviour for the maximum storable NOx mass coincides very well for the vehicle measurements and the synthetic gas test bench measurements. The NOx adsorption and desorption behaviour with a preloading of 2% H2O for 100 s is plotted in Fig. 18. A slight shift towards a higher desorption temperature can be noticed for the second desorption peak. Furthermore the H2O dosing shows no influence on the gas composition of the desorption peaks, with the first emissions peak consisting predominantly of NO and the second one mainly of NO2.
The results for the complete water adsorption test series are depicted in Fig. 19 and demonstrate that the slightest amount of water strongly inhibits the storable amount of NOx. However, during real vehicle measurements the idealized case of no water preloading will hardly occur, because in every operating condition water will reach the surface of the catalyst. Despite the strong gradient for low water dosing it is noteworthy that a kind of saturation of minimum NOx storage is reached for longer water dosing periods. This behaviour is contrary to that seen in vehicle measurements, where the NOx storage effect completely disappears as the amount of preloaded water exceeds a certain value.
6 Summary and conclusion
This paper aimed to experimentally analyse and evaluate the NOx storage behaviour as well as the water adsorption/desorption behaviour on SCR catalyst systems during vehicle cold start. In vehicle measurements the overall behaviour could be classified in the mentioned sub-effects, furthermore the effects have been replicated on a synthetic gas test bench. The lab scale investigations provide a detailed overview for each influence parameter.
The investigated SCR catalysts show a sharp temperature rise during cold start in dry condition, this effect can be traced back to the exothermic adsorption behaviour of H2O on the surface of the catalyst. A higher water pre-loading on the catalyst causes a significantly decreased temperature rise. The exothermic behaviour can be subject to a time-based decoupling, traceable to the condensation of the gaseous water upstream of cold exhaust components. In addition, the catalyst analysed is capable of adsorbing NOx emissions at low temperatures, causing zero NOx emissions after the catalyst. The temperature rise was reproduced on the synthetic gas test bench and it is proofed that the rise in temperature causes the previously adsorbed NOx to desorb. It is assumed that locally much higher temperatures are reached enabling a partial reduction of the stored NOx if NH3 is available.
Preloaded amounts of water on the catalyst and the catalyst temperature were identified to be the main influence parameters on the NOx storage behaviour with regards to the storage capacity. Additionally the impact of CO, C3H6 and C10H22 was verified, whereby a NOx reduction effect for the short- and long-chain hydrocarbons was determined. CO in the exhaust gas primarily affects the storage sites with the higher NOx release temperature by inhibiting the oxidation of NO to NO2 during the adsorption phase. C3H6 increases the reduction potential of NOx stored on the catalyst during the NOx desorption. Long-chain hydrocarbons significantly decrease the storable amount of NOx on the catalyst, due to a blocking effect of the NOx storage sites on the catalyst. Furthermore, synthetic gas test bench measurements revealed that the space velocity predominantly affects the adsorption behaviour. An increasing mass flow causes a disproportionate earlier NOx slip, due to a shorter dwell time of each NOx molecule on the catalyst. The influence of the catalyst temperature shows that a higher temperature reduces the NOx storage behaviour, therefore the NOx storage effect is available only during cold starts.
The different behaviour of a flow-through substrate and an on-filter substrate was analysed. It is assumed that, with a higher amount of washcoat the storable amount of NOx increases. The seen behaviour corresponds very well to that of vehicle measurements. Additionally the influence of stored NH3 on the catalyst was analysed, showing that the presence of NH3 reduces storable amount of NOx on the one hand, but on the other hand increases the NOx reduction potential.
Overall, it was possible to outline the main influence parameters on the NOx storage effect as well as their effect mechanisms on the synthetic test gas bench. To increase the accuracy of an already existing SCR system model, an empirical NOx storage model will be developed and implemented. This model will be able to give a prediction whether a NOx storage potential is available and if so, how much NOx reduction will occur under the given boundary conditions during the cold start phase.
Abbreviations
- ASC:
-
Ammonia slip catalyst
- C10H22 :
-
Decane
- C3H6 :
-
Propene
- CH4N2O:
-
Urea
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- DOC:
-
Diesel oxidation catalyst
- DPF:
-
Diesel particulate filter
- FTIR:
-
Fourier-transform infrared spectroscopy
- GHSV:
-
Gas hourly space velocity
- H2O:
-
Water
- HNCO:
-
Isocyanic acid
- N2 :
-
Nitrogen
- N2O:
-
Nitrous oxide
- NDIR:
-
Nondispersive infrared sensor
- NH3 :
-
Ammonia
- NO:
-
Nitrogen monoxide
- NO2 :
-
Nitrogen dioxide
- NOx :
-
Nitrogen oxides
- O2 :
-
Oxygen
- PID:
-
Proportional–integral–derivative controller
- PMA:
-
Paramagnetic analyser
- RDE:
-
Real driving emissions
- SCR:
-
Selective catalytic reduction
- TPD:
-
Temperature programmed desorption
References
ACEA. https://www.acea.be/statistics/article/Share-of-diesel-in-new-passenger-cars. Accessed 19 May 2021
Mitterecker, H., Wieser, M., Weißbäck, M., et al.: The diesel engine as an important component for CO2 fleet target achievement. MTZ Worldwide. 79, 38–43 (2018)
Reşitoğlu, İA., Altinişik, K., Keskin, A.: The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Techn. Environ. Policy 17, 15–27 (2015). https://doi.org/10.1007/s10098-014-0793-9
Schmeisser, V., Weibel, M., Sebastian Hernando, L., et al.: Cold start effect phenomena over Zeolite SCR catalysts for exhaust gas aftertreatment. SAE Int. J. Commer. Veh. 6, 190–199 (2013). https://doi.org/10.4271/2013-01-1064
Lao, C.T., Akroyd, J., Eaves, N., et al.: Investigation of the impact of the configuration of exhaust after-treatment system for diesel engines. Appl. Energy 267, 114844 (2020). https://doi.org/10.1016/j.apenergy.2020.114844
(2017) SAE Technical Paper Series. SAE Technical Paper Series. SAE International400 Commonwealth Drive, Warrendale, PA, United States
Mihai, O., Tamm, S., Stenfeldt, M., et al.: Evaluation of an integrated selective catalytic reduction-coated particulate filter. Ind. Eng. Chem. Res. 54, 11779–11791 (2015). https://doi.org/10.1021/acs.iecr.5b02392
Bayer T, Samuelsen D, Bareiss S et al. (2018) Double injection SCR–Bosch’s development for future emission regulations. In: Bargende M, Reuss H-C, Wiedemann J (eds) 18. Internationales Stuttgarter Symposium. Springer Fachmedien Wiesbaden, Wiesbaden, pp 579–593
Olowojebutu S, Steffen T (2017) A Review of the Literature on Modelling of Integrated SCR-in-DPF Systems. In: SAE Technical Paper Series. SAE International400 Commonwealth Drive, Warrendale, PA, United States
Purfürst, M., Naumov, S., Langeheinecke, K.-J., et al.: Influence of soot on ammonia adsorption and catalytic DeNO x -properties of diesel particulate filters coated with SCR-catalysts. Chem. Eng. Sci. 168, 423–436 (2017). https://doi.org/10.1016/j.ces.2017.04.052
Steinparzer F, Hiemesch D, Kranawetter E et al. (2020) Die Technik der neuen BMW 6-Zylinder-Dieselmotoren der zweiten Baukastengeneration/The Technical Concept of the New BMW 6-Cylinder 2nd Generation Modular Diesel Engines. In: Geringer B, Lenz H-P (eds) 41. Internationales Wiener Motorensymposium 22-24. April 2020. VDI Verlag, II-34-II-53
Beale, A.M., Gao, F., Lezcano-Gonzalez, I., et al.: Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem Soc Rev 44, 7371–7405 (2015). https://doi.org/10.1039/C5CS00108K
Brandenberger, S., Kröcher, O., Tissler, A., et al.: The State of the art in selective catalytic reduction of NO x by ammonia using metal-exchanged zeolite catalysts. Cataly Rev. 50, 492–531 (2008). https://doi.org/10.1080/01614940802480122
Chen, L., Janssens, T.V.W., Vennestrøm, P.N.R., et al.: A Complete Multisite Reaction Mechanism for Low-Temperature NH 3 -SCR over Cu-CHA. ACS Catal 10, 5646–5656 (2020). https://doi.org/10.1021/acscatal.0c00440
Yim, S.D., Kim, S.J., Baik, J.H., et al.: Decomposition of Urea into NH 3 for the SCR Process. Ind Eng Chem Res 43, 4856–4863 (2004). https://doi.org/10.1021/ie034052j
Ebrahimian, V., Nicolle, A., Habchi, C.: Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR systems. AIChE J 58, 1998–2009 (2012). https://doi.org/10.1002/aic.12736
Devada, M., Krocher, O., Elsener, M., et al.: Influence of NO2 on the selective catalytic reduction of NO with ammonia over Fe-ZSM5. Appl. Catal. B 67, 187–196 (2006). https://doi.org/10.1016/j.apcatb.2006.04.015
Ciardelli, C., Nova, I., Tronconi, E., et al.: Reactivity of NO/NO2–NH3 SCR system for diesel exhaust aftertreatment: Identification of the reaction network as a function of temperature and NO2 feed content. Appl. Catal. B 70, 80–90 (2007). https://doi.org/10.1016/j.apcatb.2005.10.041
Roppertz, A., Füger, S., Kureti, S.: Investigation of Urea-SCR at Low Temperatures. Top Catal 60, 199–203 (2017). https://doi.org/10.1007/s11244-016-0597-8
Chen, L., Janssens, T.V.W., Skoglundh, M., et al.: Interpretation of NH3-TPD Profiles from Cu-CHA Using First-Principles Calculations. Top Catal 62, 93–99 (2019). https://doi.org/10.1007/s11244-018-1095-y
Kwak, J.H., Tonkyn, R.G., Kim, D.H., et al.: Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 275, 187–190 (2010). https://doi.org/10.1016/j.jcat.2010.07.031
Schmieg, S.J., Oh, S.H., Kim, C.H., et al.: Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 184, 252–261 (2012). https://doi.org/10.1016/j.cattod.2011.10.034
Gao, F., Walter, E.D., Karp, E.M., et al.: Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 300, 20–29 (2013). https://doi.org/10.1016/j.jcat.2012.12.020
Lundström, A., Snelling, T., Morsing, P., et al.: Urea decomposition and HNCO hydrolysis studied over titanium dioxide, Fe-Beta and γ-Alumina. Appl. Catal. B 106, 273–279 (2011). https://doi.org/10.1016/j.apcatb.2011.05.010
Selleri, T., Gramigni, F., Nova, I., et al.: A PGM-free NO x adsorber + selective catalytic reduction catalyst system (AdSCR) for trapping and reducing NO x in lean exhaust streams at low temperature. Catal Sci Technol 8, 2467–2476 (2018). https://doi.org/10.1039/C8CY00343B
Gramigni, F., Selleri, T., Nova, I., et al.: Analysis of AdSCR Systems for NOx Removal During the Cold-Start Period of Diesel Engines. Top Catal 62, 3–9 (2019). https://doi.org/10.1007/s11244-019-01138-5
Storch JGC (2009) Materialwissenschaftliche Untersuchungen an zeolithischen Adsorbenzien für den Einsatz in offenen Sorptionssystemen zur Wärmespeicherung. Dissertation, Technische Universität München
Hauer A (2002) Beurteilung fester Adsorbentien in offenen Sorptionssystemen für energetische Anwendungen. Dissertation, Technische Hochschule Berlin
Shelef, M.: Selective catalytic reduction of NOx with N-free reductants. Chem Rev 95, 209–225 (1995). https://doi.org/10.1021/cr00033a008
Iwamoto, M., Yahiro, H., Tanda, K., et al.: Removal of nitrogen monoxide through a novel catalytic process. 1. Decomposition on excessively copper-ion-exchanged ZSM-5 zeolites. J Phys Chem 95, 3727–3730 (1991). https://doi.org/10.1021/j100162a053
Funding
Open access funding provided by Graz University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lukas, D., Michael, M., Gert, B. et al. Analysis of the NOx storage behaviour during cold start of modern SCR flow-through substrates and SCR on-filter substrates. Automot. Engine Technol. 7, 81–96 (2022). https://doi.org/10.1007/s41104-021-00099-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41104-021-00099-w