Abstract
Application of chromium and discharging of high-chromium-containing wastewater is a major concern in leather processing. In tanning process, animal hide/skin collagen is stabilized against biodegradability mostly with basic chromium sulfate. Subsequently in chrome tanning, a fraction of chromium remains in the tan liquor, which is discharged in the waste steam. The study focused on the characterization of discharged chromium-containing wastewater from the tanneries. Results indicate that high concentration of chromium ranging from 2656 to 5420 mg/L was discharged as wastewater after wet-blue production. The physical parameter of spent chrome liquor: total solids (TS) were extremely high. The chemical parameter such as pH (2.4–3.0) was highly acidic. A fraction of discharged spent chrome liquor is directly mixed with the water of the River Buriganga, which causes serious environmental pollution; another fraction of chromium is settled out in the lagoon or adsorbed by sediment/soil. It is suspected that the adsorbed chromium could be eluted to groundwater in the tannery area, which could be a great threat in the near future. Practicing chromium recovery and reuse systems might be a good approach to make leather production environmental friendly, and consequently Bangladesh could save huge amounts of chemical costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In tannery operations, animal hide/skin is received as by-product from the meat industry and is processed to make it into something useful, special, and noble. Leather processing involves the conversion of putrescible hides/skins into imputrescible leather by the process of tanning. In the tanning process, a number of chemicals and mechanical operations are carried out to produce good quality leather. In the soaking to finishing operations, a wide variety of chemicals is used. It is reported that only 15 % of offered chemicals are retained in the finished leather, while the residue of 85 % chemicals enter the waste streams (UNIDO 2000). After tanning operations, a good fraction of these chemicals is discarded as wastes in solid, liquid, or gaseous form. All these wastes may cause serious environmental pollution in water, soil, and air.
Leather and its allied industries are one of the biggest export earning sectors to strengthen the national economy of Bangladesh. Export Promotion Bureau (EPB) reported that Bangladesh earned US $1.29 billion in the fiscal year of 2013–2014 from the leather sector, 4.29 % of the total exports (EPB 2014). There are 187 tanneries located at Hazaribagh in the western part of capital city Dhaka, Bangladesh (PKF 2013). Annually about 85,000 tons of raw hide/skin are estimated to be processed for leather production in Bangladesh, which generate huge amount of solid and liquid wastes (Paul et al. 2013). Unfortunately, excluding one modern tannery (Apex Tannery, Unit-2), none of the tanneries has an effluent treatment plant (ETP). Bangladesh is facing environmental degradation of the river, Buriganga and other linked rivers due to receiving the discharged green solid and liquid wastes from the tanneries (Zahid et al. 2006).
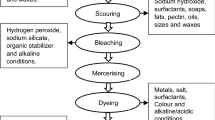
In leather processing, hide/skin has to pass through numerous operational steps. The operational sequence for the conventional wet-blue process is shown in Fig. 1. Chrome tanning is the subsequent operation of pickling and is the most common technique in leather processing; 90 % tanning of industries use basic chromium sulfate (BCS) instead of other tanning agents to obtain better quality leather (Avindhan et al. 2004). BCS binds with collagen protein to make it stabilize against biodegradation. Chromium has a tendency to precipitate at pH more than 4.0 (Thanikaivelan et al. 2000), which limits the possibility of higher penetration of chromium at pH levels (2.0–4.0) (Sharphouse 1971).
On average, only 60 % of the chromium is uptaken by the pickled pelt and the other 40 % of chromium remains in the solid and liquid wastes, especially as spent chrome liquor (Fabiani et al. 1997). It is reported that in conventional chrome tanning, wastewater contains 1500–3000 mg/L chromium (Suresh et al. 2001); it seems that huge amount of chromium content remains in the spent chrome liquor. Tanneries at Hazaribagh, Dhaka discharge their spent chrome liquor directly through the drain after wet-blue production. A fraction of discharged spent chrome liquor is directly mixed with the River Buriganga, which causes serious environmental pollution; another fraction of chromium is settled in the lagoon or adsorbed by sediment/soil. Chromium exists in several oxidation states, with trivalent Cr3+ and hexavalent Cr6+ species being the most common forms (Kotaś and Stasicka 2000). The occupational exposure of chromium has been widespread and it is shown that chromium (III) under certain ligand conditions in environments leads to cell death and structural modification of proteins (Balamurugan et al. 2002). Many countries are practicing for the recoveries and reuse system of chromium. Practicing recovery of chromium is a good approach which involves the filtration of waste liquor followed by the chemical replacement (Sharp 1981).
A good number of researchers have documented characterization of tannery wastes in Bangladesh and their impact on the environment (Azom et al. 2012; Siddiqee et al. 2012; Rouf et al. 2013; Tinni et al. 2014). In most cases, samples were collected after mixing of discharged effluent from the tanneries in the fall of the stream before discharge to the River Buriganga, from where it is difficult to identify the specific effluent state of a specific operation.
In this study, spent chrome liquor was exclusively examined after wet-blue production to determine (1) concentration of chromium, (2) pH, (3) dissolved oxygen (DO), and (4) total solids (TS). The obtained data were complied with Bangladesh standards for the tannery industry effluent (ECR 1997).
Materials and methods
Sample collection
The study area was located at Hazaribagh, Dhaka where 85 % tanneries of Bangladesh are producing leather all year round (PKF 2013). Nine medium and large category tanneries were selected for sampling of spent chrome liquor just after wet-blue production. The spent chrome liquors were collected into high-density polyethylene (HDPE) bottles, which were prewashed with dilute nitric acid and were transported to laboratory as soon as possible for experimentation. The collected samples were identified as S1, S2, S3, S4, S5, S6, S7, S8, and S9. Physical, chemical and/or biochemical reactions may take place in the sample container, which leads to change the intrinsic quality of the sample during collection of analysis in the laboratory. Therefore, for quantification of chromium content, samples were preserved with 2 mL concentrated nitric acid/L sample. All samples were kept in the refrigerator at 4 °C until the experiment was completed.
Analysis of physiochemical parameters
Experiments were carried out to quantify the levels of physiochemical parameters, such as chromium (Cr), pH, dissolved oxygen (DO), total solids (TS), total dissolved solids (TDS), and total suspended solids (TSS). The parameters, pH and dissolved oxygen (DO) are highly unstable; they were measured at the sampling site.
Determination of total chromium
After filtration of the spent chrome liquor with filter paper (Whatman No. 1), total chromium was measured by the atomic absorption spectroscopy (SpectrAA-220, VARIAN, Australia) with direct air–acetylene (air–C2H2) flame at the wavelength of 357.9 nm.
Determination of pH
pH of the samples was measured on-site by using the pH (UPH-314, UNILAB, USA) meter. Before measuring, the pH meter was calibrated by the standard solution.
Determination of dissolved oxygen
Dissolved oxygen of the samples was measured on-site by using the DO meter (HQ40d, HACH, USA). Before measuring, the DO meter was calibrated by the standard solution.
Determination of total solids
Total solids (TS), total dissolved solids (TDS) and total suspended solids (TSS) were determined gravimetrically following the standard methods of APHA (2012). A 10-mL liquid sample was passed through the glass fiber filter and dried in the drying oven at 103–105 °C until a constant weight was obtained.
Results and discussion
Total chromium concentration
The concentrations of total chromium in the spent chrome liquors are illustrated in Fig. 2. The level of chromium concentrations of the samples was 2656–5420 mg/L, which was extremely high, whereas the standard for industrial effluent of the tannery industry is 2 mg/L (ECR 1997). It seems that concentration of chromium in the spent chrome liquor was 1328–2710 times higher than the standard level.
The high-concentrated spent chrome liquor is discharged from the tanneries at Hazarigbagh, Dhaka, directly through drain without applying any alternative way to recover or reuse part of the system. Of course, a fraction of chromium is precipitated as chromic hydroxide or absorbed by soil/sediment or carried as liquid phase and fall to the low-lying adjacent areas. The chromium-containing wastewater is finally mixed with the River Buriganga, which means the linking rivers are also becoming contaminated with chromium. Under favorable conditions, chromium could be eluted from the adsorbed sediment/soil which could then be mobilized to groundwater. Primarily chromium has oxidation state Cr(III), but it could be changed to Cr(VI) under certain conditions, which is very toxic and irritating for human body tissue (Dahbi et al. 2002). It is also shown that Cr(III) under certain ligands in the environment leads to cell death and structural modification of proteins (Balamurugan et al. 2002).
pH level in the spent chrome liquors
Figure 3 shows the pH values of spent chrome liquor from different tanneries. The pH levels varied from 2.4 to 3.0. It seems that pH levels of all samples were beyond the standard (pH 6–9) for industrial effluent of tannery industry (ECR 1997).
Usually before chrome tanning, in the pickling stage, float pH is maintained at 2.5–3.0 for better penetration of the chrome into pelts (Covington 2011). After sufficient penetration of chrome tanning agents, the pH is generally raised ~4 to fix chromium with collagen. After completing chrome tanning, spent chrome liquor is discharged at lower pH (2.3–4.0) without neutralization. In Hazaribagh, generally all variety of wastewaters from tanning operations is discharged through a common drainage system, which finally comes together. Wastewater containing sulphide, upon acidification (8.0 < pH) sulphide is emitted as hydrogen sulphide (H2S) gas (Dixit et al. 2015). During the transport of the spent chrome liquor through drain, it mixes with simultaneously discharged lime liquor (pH 12.0–13.5), resulting in the production of toxic hydrogen sulphide (H2S) gas, which causes an environmental nuisance. Likewise, high acidic spent chrome liquor decreases the pH level in adjacent low-lying areas of aquatic life before it finally falls to the River Buriganga.
Dissolved oxygen level in the spent chrome liquors
Figure 4 represents the dissolved oxygen (DO) levels of the measured samples. The DO values range between 2.6 and 8.3 mg/L; even though the samples were from the same operations, they come from different tanneries. The dissolved oxygen standard for waste from industrial units is 4.5–8.0 mg/L (ECR 1997). The DO values for S3 and S7 are close to lower boarder limits and only S1 and S2 DO levels tended to upper boarder limits. For the rest of the samples, DO values were below the standard level.
The spent chrome liquors with lower DO values are directly discharged to drain to fall to the low-lying area and finally are mixed with the river water of Buriganga. Liquid waste from various operations as well as from different tanneries fall to River Buriganga, which is one of the most common reasons for the decreasing DO levels. As a result, numbers of fish as well as aquatic plants are disappearing due to lack of dissolved oxygen.
Total solids (TS)
The level of total solids (TS), total dissolved solids (TDS), and total suspended solids (TSS) are shown in Table 1. Total suspended solids were extremely high and beyond the permissible level. TSS standard level for tannery industry effluent is 150 mg/L (ECR 1997) but the experimental values were 36–95 times higher than the standard level. TDS standard level for tannery industry effluent (ECR 1997) is 2100 mg/L but the tested results were 22–53 times higher than the standard level. The total solids have a negative effect on aquatic life. The discharged suspended matters get deposited on the bed of the stream and kills aquatic organisms who dwell on the stream bottom. The floating solids interfere with the stream’s ability for self-purification by re-generation of the oxygen absorption from the atmosphere. TS also interfere with the photosynthesis activity of the stream plankton and aquatic plants. Suspended solids cause turbidity that decreases infiltration of sunlight into water, thus reducing the photosynthesis activity of aquatic plants.
Conclusions
Every year, huge amounts of spent chrome liquor are discharged from the tanneries after wet-blue production as wastewater through the drain to low-lying areas and finally to the River Buriganga. The resulting pH, DO and TS values have negative effect on aquatic life. Chromium is introduced in the form of Cr(III) which has less negative impact, but it could be changed as Cr(VI) which is carcinogenic. Due to lack of dissolved oxygen, aquatic living organisms including fish are disappearing. The higher TS and lower pH also have negative effect on the aquatic life. All concerned authorities, especially tannery owners, should practice recovery and reuse systems for chromium to lessen these environment impacts; consequently chemical cost could also be minimized.
References
Accountants and Business Advisers (PKF) (2013) Technical report: leather sector includes a value chain analysis and proposed action plans. Bangladesh INSPIRED, Dhaka
American Public Health Association (APHA) (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC
Avindhan R, Madhan B, Raghava Rao J, Unni Nair B, Amasami T (2004) Bioaccumulation of chromium from tannery wastewater: an approach for chrome recovery and reuse. Environ Sci Technol 38:300–306
Azom MR, Mahmud K, Yahya SM, Sontu A, Himon SB (2012) Environmental impact assessment of tanneries: a case study of Hazaribag in Bangladesh. Int J Environ Sci Develop 3(2):152–156
Balamurugan K, Rajaram R, Ramasami T, Narayanan S (2002) Chromim(III) induced apoptosis of lymphocytes: death decision by ROS and Src-family tyrosine kinases. Free Radic Biol Med 33:1622–1640
Covington AD (2011) Tanning chemistry: the science of leather. RSC Publishing, UK 127
Dahbi S, Azzi M, Saib N, Guardia M, Faure R, Durand R (2002) Removal of trivalent chromium from tannery waste waters using bone charcoal. Anal Bioanal Chem 374:540–546
Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87:39–49
Export Promotion Bureau (EPB) (2014) Ministry of Commerce, Bangladesh
Fabiani C, Ruscio F, Spadoni M, Pizzichini M (1997) Chromium(III) salts recovery process from tannery wastewaters. Desalination 108:183–191
Kotaś J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107:263–283
Paul HL, Antunes APM, Covington AD, Evans P, Phillips PS (2013) Bangladeshi leather industry: an overview of recent sustainable developments. J Soc Leather Technol Chem 97(1):25–32
Rouf MA, Islam MS, Haq MZ, Ahmed N, Rabeya T (2013) Characterization of effluents of leather industries in Hazaribagh area of Dhaka city. Bangladesh J Sci Ind Res 48:155–166
Sharp BW (1981) Chrome recycling. J Am Leather Chem Assoc 76:24–34
Sharphouse JH (1971) Leather technicians handbooks. Leather Products Association, Northampton 72
Siddiqee MH, Islam MS, Rahman MM (2012) Assessment of pollution caused by tannery-waste and its impact on aquatic bacterial community in Hajaribag, Dhaka. Stamford J Microbiol 2(1):20–23
Suresh V, Kanthimathi M, Thanikaivelan P, Rao JR, Nair BU (2001) An improved product-process for cleaner chrome tanning in leather processing. J Clean Prod 9:483–491
Thanikaivelan P, Geetha V, Rao JR, Sreeram KJ, Nair BU (2000) A novel chromium-iron tanning agent: cross-fertilisation in solo tannage. J Soc Leather Technol Chem 84:82–87
The Environment Conservation Rules (1997) Ministry of Environment and Forest, Bangladesh
Tinni SH, Islam MA, Fatima K, Ali MA (2014) Impact of tanneries waste disposal on environment in some selected areas of Dhaka City Corporation. J Environ Sci Nat Resour 7(1):149–156
United Nations Industrial Development Organization (UNIDO) (2000) Chrome balance in leather processing, pp 1
Zahid A, Balke K-D, Hassan MQ, Flegr M (2006) Evaluation of aquifer environment under Hazaribagh leather processing zone of Dhaka city. Environ Geol 50:495–504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashem, M.A., Islam, A., Mohsin, S. et al. Green environment suffers by discharging of high-chromium-containing wastewater from the tanneries at Hazaribagh, Bangladesh. Sustain. Water Resour. Manag. 1, 343–347 (2015). https://doi.org/10.1007/s40899-015-0033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-015-0033-4