Abstract
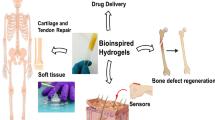
This review aims to present methods for obtaining polysaccharide hydrogels, their properties and sensitivity to environmental stimuli, as well as their potential applications in biomedicine. Living systems respond to external stimuli by adapting themselves to changing conditions. Hydrogels are a class of materials with 3D networks of polymers that can absorb high amounts of water or biological fluids while remaining insoluble under physiological conditions compared with general absorbent materials, with their characteristic being dependent on network structure and the external environment. Stimuli-responsive hydrogels have the ability to respond to changes in their external environment. They can exhibit dramatic changes in their swelling behavior, network structure, permeability, and mechanical strength in response to variations in temperature, pH, glucose, electric field, light, etc. However, such changes are reversible; therefore, hydrogels can convert to their initial state as soon as the trigger is removed. Because of compatibility with living tissues, hydrogels can be used in different biomedical purposes.
Lay Summary
The application of stimuli-responsive polysaccharide hydrogels in the biomedical field has become increasingly popular with many research groups and industries. In addition to their ability to undergo large reversible transitions in their swelling behavior due to small physiological or environmental changes, they are also often highly biocompatible and versatile and possess a high storage capacity for the immobilization of biomolecules.













Similar content being viewed by others
References
Rasoulzadeh M, Namazi H. Carboxymethyl cellulose/graphene oxide bionanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym. 2017;168:320–6.
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int J Biol Macromol. 2015;73:109–14.
Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18–23.
Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–8.
Gholamali I, Hosseini SN, Alipour E, Yadollahi M. Preparation and characterization of oxidized starch/CuO nanocomposite hydrogels applicable in a drug delivery system. Starch/Stärke. 2019;71(3-4).
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46.
Ullah F, Othman MBH, Javed F, Ahmad Z, Akil HM. Classification, processing and application of hydrogels: a review. Mater Sci Eng C. 2015;57:414–33.
Ganji F, Vasheghani-Farahani S, Vasheghani-Farahani E. Theoretical description of hydrogel swelling: a review. Iran Polym J. 2010;19(5):375–98.
Das N. Preparation methods and properties of hydrogel: a review. J Pharm Pharm Sci. 2013;5(3):112–7.
Malmsten M. Antimicrobial and antiviral hydrogels. Soft Matter. 2011;7:8725–36.
Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–408.
Richter A, Paschew G, Klatt S, Lienig J, Arndt KF, Adler HJP. Review on hydrogel-based pH sensors and microsensors. Sensors. 2008;8:561–81.
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Tambourgi EB. Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem Eng J. 2011;168:68–76.
Bakravi A, Ahamadian Y, Hashemi H, Namazi H. Synthesis of gelatin-based biodegradable hydrogel nanocomposite and their application as drug delivery agent. Adv Polym Technol. 2018;37:2625–35.
Venkatesan J, Lowe B, Pallela R, Kim SK. Chitosan-based polysaccharide biomaterials. Polysaccharides. 2015:1837–50.
Basu A, Kunduru KR, Abtew E, Domb AJ. Polysaccharide-based conjugates for biomedical applications. Bioconjug Chem. 2015;26(8):1396–412.
Kabiri R, Namazi H. Synthesis of cellulose/reduced graphene oxide/polyaniline nanocomposite and its properties. Int J Polym Mater Polym Biomater. 2016;65:675–82.
Ul-Islam M, Khattak WA, Ullah MW, Khan S, Park JK. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose. 2014;21:433–47.
Khan S, Ul-Islam M, Khattak WA, Ullah MW, Park JK. Bacterial cellulose-titanium dioxide nanocomposites: nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose. 2015;22:565–79.
Kamel S, Ali N, Jahangir K, Shah SM, El-Gendy AA. Pharmaceutical significance of cellulose: a review. Express Polym Lett. 2008;2(11):758–78.
Klemm D, Heublein B, Fink HP. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem. 2005;44(22):3358–93.
Suhas, Gupta VK, Carrott PJM, Singh R, Chaudhary M, Kushwaha S. Cellulose: a review as natural, modified and activated carbon adsorbent. Bioresour Technol. 2016;126:1066–76.
Yadollahi M, Namazi H. Synthesis and characterization of carboxymethyl cellulose/layered double hydroxide nanocomposites. J Nanopart Res. 2013;15:1563–72.
Rakhshaei R, Namazi H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater Sci Eng C. 2017;73:456–64.
Zare-Akbari Z, Farhadnejad H, Furughi-Nia B, Abedin S, Yadollahi M, Khorsand GM. pH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int J Biol Macromol. 2016;93:1317–27.
Yadollahi M, Namazi H, Aghazadeh M. Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int J Biol Macromol. 2015;79:269–77.
Basta AH, El-Saied H. New approach for utilization of cellulose derivatives metal complexes in preparation of durable and permanent colored papers. Carbohydr Polym. 2008;74(2):301–8.
Shen J, Song Z, Qian X, Yang F. Carboxymethyl cellulose/alum modified precipitated calcium carbonate fillers: preparation and their use in papermaking. Carbohydr Polym. 2010;81(3):545–53.
Choi Y, Simonsen J. Cellulose nanocrystal filled carboxymethyl cellulose nanocomposites. J Nanosci Nanotechnol. 2006;6(3):633–9.
Luna-Martinez JF, Hernandez-Uresti DB, Reyes-Melo ME, Guerrero-Salazar CA, Gonzalez-Gonzalez VA, Sepulveda-Guzman S. Synthesis and optical characterization of ZnS-sodium carboxymethyl cellulose nanocomposite films. Carbohydr Polym. 2011;84(1):566–70.
Foroutan R, Ahmadlouydarab M, Ramavandi B, Mohammadi R. Studying the physicochemical characteristics and metals adsorptive behavior of CMC-g-HAp/Fe3O4 nanobiocomposite. J Environ Chem Eng. 2018;6:6049–58.
Yadollahi M, Namazi H, Barkhordari S. Preparation and properties of carboxymethyl cellulose/layered doublehydroxide bionanocomposite films. Carbohydr Polym. 2014;108:83–9.
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M. Synthesis and characterization of antibacterial carboxymethylcellulose/ZnO nanocomposite hydrogels. Int J Biol Macromol. 2015;74:136–41.
Hebeish A, Hashem M, Abd El-Hady MM, Sharaf S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohydr Polym. 2013;92:407–13.
Ward MA, Georgiou TK. Thermoresponsive polymers for biomedical applications. Polym. 2011;3(3):1215–42.
Jyoti BVS, Baek SW. Formulation and comparative study of rheological properties of loaded and unloaded ethanol-based gel propellants. J Energ Mater. 2015;33:125–39.
McAllister JW, Lott JR, Schmidt PW, Sammler RL, Bates FS, Lodge TP. Linear and nonlinear rheological behavior of fibrillar methylcellulose hydrogels. ACS Macro Lett. 2015;4:538–42.
Picheth GF, Pirich CL, Sierakowski MR, Woehl MA, Sakakibara CN, De Souza CF, et al. Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol. 2017;104:97–106.
De Oliveira SA, Da Silva BC, Riegel-Vidotti IC, Urbano A, De Sousa Faria-Tischer PC, Tischer CA. Production and characterization of bacterial cellulose membranes with hyaluronic acid from chicken comb. Int J Biol Macromol. 2017;97:642–53.
Hayashi N, Kondo T, Ishihara M. Enzymatically produced nano-ordered short elements containing cellulose I-beta crystalline domains. Carbohydr Polym. 2005;61(2):191–7.
Abe K, Iwamoto S, Yano H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules. 2007;8(10):3276–8.
Paakko M, Ankerfors M, Kosonen H, Nykanen A, Ahola S, Osterberg M, et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules. 2007;8(6):1934–41.
Nakagaito AN, Yano H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl Phys A Mater. 2004;78(4):547–52.
Jasim A, Ullah MW, Shi Z, Lin X, Yang G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr Polym. 2017;164:214–21.
Andrade FK, Alexandre N, Amorim I, Gartner F, Mauricio AC, Luis AL. Studies on the biocompatibility of bacterial cellulose. J Bioact Compat Polym. 2013;28:97–112.
Avila HM, Feldmann EM, Pleumeekers MM, Nimeskern L, Kuo W, De Jong WC, et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials. 2015;44:122–33.
Rajwade JM, Paknikar KM, Kumbhar JV. Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol. 2015;99:2491–511.
Mishra RK, Banthia AK, Majeed ABA. Pectin based formulations for biomedical applications: a review. Asian J Pharm Clin Res. 2012;5:1–7.
Liu L, Fishman ML, Hicks KB. Pectin in controlled drug delivery: a review. Cellulose. 2007;14:15–24.
Ranjha NM, Mudassir J, Sheikh ZZ. Synthesis and characterization of pH-sensitive pectin/acrylic acid hydrogels for verapamil release study. Iran Polym J. 2011;20:147–59.
Sudheesh Kumar PT, Lakshmanan VK, Biswas R, Nair SV, Jayakumar R. Synthesis and biological evaluation of chitin hydrogel/Nano ZnO composite bandage as antibacterial wound dressing. J Biomed Nanotechnol. 2012;8:1–10.
Zargar V, Asghari M, Dashti A. A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. Chem Biol Eng Rev. 2015;2:1–24.
Kurita K. Controlled Functionalization of the polysaccharide chitin. Progress Polym Sci. 2001;26:1921–71.
Tamura H, Nagahama H, Tokura S. Preparation of chitin hydrogel under mild conditions. Cellulose. 2006;13(4):357–64.
Copello GJ, Mebert AM, Raineri M, Pesenti MP, Diaz LE. Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol-gel method. J Hazard Mater. 2011;186:932–9.
Barikani M, Oliaei E, Seddiqi H, Honarkar H. Preparation and application of chitin and its derivatives: a review. Iran Polym J. 2014;23:307–26.
Sharp RG. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy. 2013;3(4):757–93.
Anitha A, Sowmya S, Sudheesh Kumar PT, Deepthi S, Chennazhi KP, Ehrlich H, et al. Chitin and chitosan in selected biomedical applications. Prog Polym Sci. 2014;39:1644–67.
Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater Sci Eng C. 2018;87(1):50–9.
Farhoudian S, Yadollahi M, Namazi H. Facile synthesis of antibacterial chitosan/CuO bio-nanocomposite hydrogel beads. Int J Biol Macromol. 2016;82:837–43.
Rasoulzadehzali M, Namazi H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int J Biol Macromol. 2018;116:54–63.
Gholamali I, Asnaashariisfahani M, Alipour E. Silver nanoparticles incorporated in pH-sensitive nanocomposite hydrogels based on carboxymethyl chitosan-poly (vinyl alcohol) for use in a drug delivery system. Regen Eng Transl Med. 2019:1–16.
Panchal V, Vyas B, Chauhan CS, Goyal PK, Sarangdevot YS. Chitosan as a natural polymer: an overview. www.pharmaerudition.org. 2015;5(2):1-8.
Wu J, Hou S, Ren D, Mather PT. Antimicrobial properties of nanostructured hydrogel webs containing silver. Biomacromolecules. 2009;10:2686–93.
Yadollahi M, Farhoudian S, Namazi H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2015;79:37–43.
Yadollahi M, Farhoudian S, Barkhordari S, Gholamali I, Farhadnejad H, Motasadizadeh H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2016;82:273–8.
George M, Abraham TE. pH sensitive alginate-guar gum hydrogel for the controlled delivery of protein drugs. Int J Pharm. 2007;335:123–9.
Bouropoulos N, Stampolakis A, Mouzakis DE. Dynamic mechanical properties of calcium alginate-hydroxyapatite nanocomposite hydrogels. Sci Adv Mater. 2010;2:239–42.
Mohamed SF, Mahmoud GA, Abou Taleb MF. Synthesis and characterization of poly (acrylic acid)-g-sodium alginate hydrogel initiated by gamma irradiation for controlled release of chlortetracycline HCl. Monatsh Chem. 2013;144(2):129–37.
Mørch YA, Donati I, Strand BL, Bræk GS. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–80.
Paques JP, van der Linden E, van Rijn CJM, Sagis LMC. Preparation methods of alginate nanoparticles. Adv Colloid Interface. 2014;209:163–71.
Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK. Alginate composites for bone tissue engineering: a review. Int J Biol Macromol. 2015;72:269–81.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26.
Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33:3279–305.
Aljohani WJ, Wenchao L, Ullah MW, Zhang X, Yang G. Application of sodium alginate hydrogel. J Biotechn Biochem. 2017;3(3):19–31.
Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym. 2013;92:1262–79.
Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56.
Zohuriaan-Mehr MJ, Kabiri K. Superabsorbent polymer materials: a review. Iran Polym J. 2008;17(6):451–77.
Del Valle LJ, Díaz A, Puiggalí J. Hydrogels for biomedical applications: cellulose, chitosan, and protein/peptide derivatives. Gels. 2017;3:27–55.
Haraguchi K. Stimuli-responsive nanocomposite gels. Colloid Polym Sci. 2011;289:455–73.
Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64:49–60.
Gupta AK, Siddiqui AW. Environmental responsive hydrogels: a novel approach in drug delivery system. J Drug Deliv Ther. 2012;2(1):81–8.
Kashyap N, Kumar N, Kumar MR. Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug. 2005;22:107–50.
Qureshi D, Nayak SK, Maji S, Anis A, Kim D, Pal K. Environment sensitive hydrogels for drug delivery applications. Eur Polym J. 2019.
Pa’e N, Salehudin MH, Diana Hassan N, Mohd Marsin A, Idayu Muhamad I. Thermal behavior of bacterial cellulose based hydrogels with other composites and related instrumental analysis. Cellulose-Based Superabsorbent Hydrogels PP. 2018; pp. 1-25.
Wei W, Hu X, Qi X, Yu H, Liu Y, Li J, et al. A novel thermo-responsive hydrogel based on salecan and poly (N-isopropylacrylamide): synthesis and characterization. Colloid Surface B. 2015;125:1–11.
Tan H, Ramirez CM, Miljkovic N, Li H, Rubin JP, Marra KG. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–53.
Ha DI, Lee SB, Chong MS, Lee YM, Kim SY, Park YH. Preparation of thermoresponsive and injectable hydrogels based on hyaluronic acid and poly-(N-isopropylacrylamide) and their drug release behaviors. Macromol Res. 2006;14:87–93.
Ganji F, Abdekhodaie MJ. Chitosan-g-PLGA copolymer as a thermosensitive membrane. Carbohydr Polym. 2010;80:740–6.
Taylor MJ, Tomlins P, Sahota TS. Thermoresponsive gels. Gels. 2017;3:1–31.
Bai Y, Zhang Z, Zhang A, Chen L, He C, Zhuang X, et al. Novel thermo- and pH-responsive hydroxypropyl cellulose- and poly (L-glutamic acid)-based microgels for oral insulin controlled release. Carbohydr Polym. 2012;89:1207–14.
Thirumala S, Gimble JM, Devireddy RV. Methylcellulose based thermally reversible hydrogel system for tissue engineering applications. Cells. 2013;3:460–75.
Cochis A, Grad S, Stoddart MJ, Fare S, Altomare L, Azzimonti B, et al. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci Rep. 2017;7:1–12.
Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4:1–15.
Cirillo G, Spataro T, Curcio M, Spizzirri UG, Nicoletta FP, Picci N, et al. Tunable thermo-responsive hydrogels: synthesis, structural analysis and drug release studies. Mater Sci Eng C. 2015;48:499–510.
Zhang K, Wu XY. Temperature and pH-responsive polymeric composite membranes for controlled delivery of proteins and peptides. Biomaterials. 2004;25:5281–91.
Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2004;7:473–80.
Cirillo G, Nicoletta FP, Curcio M, Spizzirri UG, Picci N, Iemma F. Enzyme immobilization on smart polymers: catalysis on demand. React Funct Polym. 2014;83:62–9.
Dwivedi S, Khatri P, Mehra GR, Kumar V. Hydrogel—a conceptual overview. Int J Pharm Biol Arch. 2011;2(6):1588–97.
Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7:569–79.
Wang T, Turhan M, Gunasekaran S. Selected properties of pH-sensitive, biodegradable chitosan-poly (vinyl alcohol) hydrogel. Polym Int. 2004;53:911–8.
Javanbakht S, Nazari N, Rakhshaei R, Namazi H. Cu-crosslinked carboxymethylcellulose/naproxen/graphene quantum dot nanocomposite hydrogel beads for naproxen oral delivery. Carbohydr Polym. 2018;195(1):453–9.
Zakhireh S, Mahkam M, Yadollahi M, Jafarirad S. Investigation of pH-sensitive galactopyranoside glycol hydrogels as effective vehicles for oral drug delivery. J Polym Res. 2014;21:398–403.
Barkhordari S, Yadollahi M, Namazi H. pH sensitive nanocomposite hydrogel beads based on carboxymethyl cellulose/layered double hydroxide as drug delivery systems. J Polym Res. 2014;21:454–62.
Yang J, Chen J, Pan D, Wan Y, Wang Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr Polym. 2013;92:719–25.
Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32(4):693–710.
Dargaville TR, Farrugia BL, Broadbent JA, Pace S, Upton Z, Voelcker NH. Sensors and imaging for wound healing: a review. Biosens Bioelectron. 2013;41:30–42.
Zhang Y, Liu Z, Swaddiwudhipong S, Miao H, Ding Z, Yang Z. pH-sensitive hydrogel for micro-fluidic valve. J Funct Biomater. 2012;3:464–79.
Li X, Fu M, Wu M, Zhang C, Deng X, Dhinakar A, et al. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017;51:294–303.
Guiseppi-Elie A, Brahim SI, Narinesingh D. A chemically synthesized artificial pancreas: release of insulin from glucose-responsive hydrogels. Adv Mater. 2002;14:743–6.
Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci. 2010;35:278–301.
Egawa Y, Seki T, Takahashi S, Anzai J. Electrochemical and optical sugar sensors based on phenylboronic acid and its derivatives. Mater Sci Eng C. 2011;31:1257–64.
Albin G, Horbett TA, Ratner BD. Glucose sensitive membranes for controlled delivery of insulin: insulin transport studies. J Control Release. 1985;2:153–64.
Marek SR, Peppas NA. Insulin release dynamics from poly (diethylaminoethyl methacrylate) hydrogel systems. AIChE J. 2013;59:3578–85.
Wong JH, Ng TB. Isolation and characterization of a glucose/mannose/rhamnose-specific lectin from the knife bean Canavalia gladiate. Arch Biochem Biophys. 2005;439:91–8.
Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132:2–11.
Valuev IL, Vanchugova LV, Valuev LI. Glucose-sensitive hydrogel systems. Polym Sci Ser A. 2011;53(5):385–9.
Obaidat AA, Park K. Characterization of glucose dependent gel-sol phase transition of the polymeric glucose-concanavalin A hydrogel system. Pharm Res. 1996;13(7):989–95.
Yin R, Wang K, Han J, Nie J. Photo-crosslinked glucose-sensitive hydrogels based on methacrylate modified dextran-concanavalin A and PEG dimethacrylate. Carbohydr Polym. 2010;82(2):412–8.
Aslan K, Lakowicz JR, Geddes CD. Tunable plasmonic glucose sensing based on the dissociation of Con A-aggregated dextran-coated gold colloids. Anal Chim Acta. 2004;517:139–44.
Zhang C, Losego MD, Braun PV. Hydrogel-based glucose sensors: effects of phenylboronic acid chemical structure on response. Chem Mater. 2013;25(15):3239–50.
Hisamitsu I, Kataoka K, Okano T, Sakurai Y. Glucose-responsive gel from phenylborate polymer and poly (vinyl alcohol): prompt response at physiological pH through the interaction of borate with amino group in the gel. Pharm Res. 1997;14(3):289–93.
Kim A, Mujumdar SK, Siegel RA. Swelling properties of hydrogels containing phenylboronic acids. Chemosensor. 2014;2:1–12.
Nilsen-Nygaard J, Strand SP, Varum KM, Draget KI, Nordgard CT. Chitosan: gels and interfacial properties. Polym. 2015;7:552–79.
Zhang H, Wu S, Tao Y, Zhang L, Su Z. Preparation and characterization of water-soluble chitosan nanoparticles as protein delivery system. J Nanomater. 2011;2010:1–6.
Webber MJ, Anderson DG. Smart approaches to glucose-responsive drug delivery. J Drug Target. 2015;23(7-8):651–5.
Willner I. Stimuli-controlled hydrogels and their applications. Acc Chem Res. 2017;50:657–8.
Abureesh MA, Oladipo AA, Gazi M. Facile synthesis of glucose-sensitive chitosan-poly (vinyl alcohol) hydrogel: drug release optimization and swelling properties. Int J Biol Macromol. 2016;90:75–80.
Jeong B, Gutowska A. Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002;20:305–11.
Attaran A, Brummund J, Wallmersperger T. Modeling and simulation of the bending behavior of electrically-stimulated cantilevered hydrogels. Smart Mater Struct. 2015;24(3):1–15.
Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Carbohydr Polym. 2017;163:62–9.
Li H, Luo R, Lam KY. Modeling of ionic transport in electric-stimulus-responsive hydrogels. J Membr Sci. 2007;289:284–96.
Wallmersperger T, Attaran A, Keller K, Brummund J, Guenther M, Gerlach G. Modeling and simulation of hydrogels for the application as bending actuators. Progr Colloid Polym Sci. 2013;140:189–204.
Yuan Z, Li H. Modeling development and numerical simulation of transient nonlinear behaviors of electric-sensitive hydrogel membrane under an external electric field. J Biochip Tissue Chip. 2013;3:1–13.
Rahimi N, Dera R, Van den Akker NMS, Gagliardi M, Swennen G, Diliën H, Cleij, T, Post MJ, Molin DGM. Electro-responsive hydrogels for biomedical applications. Biomedicasummit.com. 2015.
Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33:1088–118.
Murdan S. Electro-responsive drug delivery from hydrogels. J Control Release. 2003;92:1–17.
Liu Y, Yan K, Jiang G, Xiong Y, Du Y, Shi X. Electrical signal guided ibuprofen release from electrodeposited chitosan hydrogel. Int J Polym Sci. 2014;2014:1–8.
Peng L, Liu Y, Huang J, Li J, Gong J, Ma J. Microfluidic fabrication of highly stretchable and fast electro-responsive graphene oxide/polyacrylamide/alginate hydrogel fibers. Eur Polym J. 2018;103:335–41.
Liu Y, Servant A, Guy OJ, Al-Jamal KT, Williams PR, Hawkins KM, et al. An electric-field responsive microsystem for controllable miniaturised drug delivery applications. Procedia Eng. 2011;25:984–7.
Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60(15):1638–49.
Takahashi SH, Lira LM. Córdoba de Torresi SI. Zero-order release profiles from a multistimuli responsive electro-conductive hydrogel. J Biomater Nanobi. 2012;3:262–8.
Tiitu M, Hiekkataipale P, Kainen JH, Makela T, Ikkala O. Viscoelastic and electrical transitions in gelation of electrically conducting polyaniline. Macromolecules. 2002;35:5212–7.
Qin XH, Ovsianikov A, Stampfl J, Liska R. Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMat. 2014;15(3-4):49–70.
Katz JS, Burdick JA. Light-responsive biomaterials: development and applications. Macromol Biosci. 2010;10:339–48.
Ilić-Stojanović S, Nikolić L, Nikolić V, Petrović S, Stanković M, Mladenović-Ranisavljević I. Stimuli-sensitive hydrogels for pharmaceutical and medical applications. Facta universitatis-series: Phys Chem Technol. 2011;9(1):37–56.
Suzuki A, Tanaka T. Phase transition in polymer gels induced by visible light. Nature. 1990;346:345–7.
Schiphorst J, Coleman S, Stumpel JE, Azouz AB, Diamond D, Schenning APHJ. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem Mater. 2015;27:5925–31.
Meng H, Hu J. A brief review of stimulus active polymers responsive to thermal, light, magnetic, electric and water/solvent stimuli. J Intell Mater Syst Struct. 2010;21:859–85.
Javvaji V, Baradwaj AG, Payne GF, Raghavan SR. Light-activated ionic gelation of common biopolymers. Langmuir. 2011;27:12591–6.
Higham AK, Bonino CA, Raghavan SR, Khan SA. Photo-activated ionic gelation of alginate hydrogel: real-time rheological monitoring of the two-step crosslinking mechanism. Soft Matter. 2014;10:4990–5002.
Lin MC, Tai HY, Ou TC, Don TM. Preparation and characterization of UV-sensitive chitosan for UV-cure with poly (ethylene glycol) dimethacrylate. Cellulose. 2012;19:1689–700.
Monier M, Abdel-Latif DA, Ji HF. Synthesis and application of photo active carboxymethyl cellulose derivatives. React Funct Polym. 2016;102:137–46.
Cohen Stuart MA, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, et al. Emerging applications of stimuli-responsive polymer materials nature materials. Nat Mater. 2010;9:101–13.
Lee KK, Cussler E, Marchetti M, McHugh MA. Pressure-dependent phase transitions in hydrogels. Chem Eng Sci. 1990;45(3):766–7.
Mahkam M. Modification of nano alginate-chitosan matrix for oral delivery of insulin. Nat Sci. 2009;7(8):1–7.
Peppas NA, Khare AR. Preparation, structure and diffusional behavior of hydrogel in controlled release. Adv Drug Deliv Rev. 1993;11:1–35.
Ferreira NN, Ferreira LMB, Cardoso VMO, Boni FI, Souza ALR, Gremião MPD. Recent advances in smart hydrogels for biomedical applications: from self-assembly to functional approaches. Eur Polym J. 2018;99:117–33.
Wang W, Kang Y, Wang A. One-step fabrication in aqueous solution of a granular alginate-based hydrogel for fast and efficient removal of heavy metal ions. J Polym Res. 2013;20:101–10.
Fernandez-Ferreiro A, Gonzalez Barcia M, Gil-Martinez M, Vieites-Prado A, Lema I, Argibay B, et al. In vitro and in vivo ocular safety and eye surface permanence determination by direct and magnetic resonance imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur J Pharm Biopharm. 2015;94:342–51.
Gambhire S, Bhalerao K, Singh S. In situ hydrogel: different approaches to ocular drug delivery. Int J Pharm Pharm Sci. 2013;5(2):27–36.
Park TG, Hoffman AS. Sodium chloride-induced phase transition in nonionic poly (N-isopropylacrylamide) gel. Macromolecules. 1993;26:5045–8.
Gawel K, Barriet D, Sletmoen M, Stokke BT. Responsive hydrogels for label-free signal transduction within biosensors. Sensors. 2010;10:4381–409.
Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19–34.
Mao J, Kondu S, Ji HF, McShane MJ. Study of the near-neutral pH-sensitivity of chitosan/gelatin hydrogels by turbidimetry and microcantilever deflection. Biotechnol Bioeng. 2006;95(3):333–41.
Beaune G, Ménager C. In situ precipitation of magnetic fluid encapsulated in giant liposomes. J Colloid Interface Sci. 2010;343(1):396–9.
Li Y, Huang G, Zhang X, Li B, Chen Y, Lu T, et al. Magnetic hydrogels and their potential biomedical applications. Adv Funct Mater. 2012;23(6):660–72.
Gil S, Mano JF. Magnetic composite biomaterials for tissue engineering. Biomater Sci. 2014;2:812–8.
Medeiros SF, Santos AM, Fessi H, Elaissari A. Stimuli-responsive magnetic particles for biomedical applications. Int J Pharm. 2011;403:139–61.
Davaran S, Alimirzalu S, Nejati-Koshki K, Tayefi Nasrabadi H, Akbarzadeh A, Khandaghi AA, et al. Physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly (N isopropylacrylamide) for anti-cancer drug delivery. Asian Pac J Cancer P. 2014;15(1):49–54.
Sriplai N, Mongkolthanaruk W, Eichhorn SJ, Pinitsoontorn S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr Polym. 2018;192:251–62.
El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. J Nanomater. 2011;2011:1–13.
Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111(3):441–53.
Chatterjee J, Haik Y, Ching JC. Modification and characterization of polystyrene-based magnetic microspheres and comparison with albumin-based magnetic microspheres. J Magn Magn Mater. 2001;225(1-2):21–9.
Reddi AH, Becerra J, Andrades JA. Nanomaterials and hydrogel scaffolds for articular cartilage regeneration. Tissue Eng B Rev. 2011;17(5):301–5.
Jun HW, Yuwono V, Paramonov SE, Hartgerink JD. Enzyme-mediated degradation of peptide amphilic nanofiber networks. Adv Mater. 2005;17:2612–7.
Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 2007;19(6):418–26.
Liu TY, Hu SH, Liu DM, Chen SY, Chen IW. Biomedical nanoparticle carriers with combined thermal and magnetic responses. Nano Today. 2009;4:52–65.
Wu J, Jiang W, Tian R, Shen Y, Jiang W. Facile synthesis of magnetic-/pH-responsive hydrogel beads based on Fe3O4 nanoparticles and chitosan hydrogel as MTX carriers for controlled drug release. J Biomater Sci Polym E. 2016;27(15):1553–68.
Zhao YZ, Du LN, Lu CT, Jin YG, Ge SP. Potential and problems in ultrasound-responsive drug delivery systems. Int J Nanomedicine. 2013;8:1621–33.
Norris P, Noble M, Francolini I, Vinogradov A, Stewart P, Ratner B, et al. Ultrasonically controlled release of ciprofloxacin from self-assembled coatings on poly (2-Hydroxyethyl methacrylate) hydrogels for Pseudomonas aeruginosa biofilm prevention. Agents Chemother. 2005;49(10):4272–9.
Uesugi Y, Kawata H, Saito Y, Tabata Y. An ultrasound-responsive nano delivery system of tissue-type plasminogen activator for thrombolytic therapy. J Control Release. 2010;147(2):269–77.
You JO, Almeda D, Ye JCG, Auguste DT. Bioresponsive matrices in drug delivery. J Biol Eng. 2010;4:15–27.
Peteu SF, Oancea F, Sicuia OA, Constantinescu F, Dinu S. Responsive polymers for crop protection. Polymers. 2010;2:229–51.
Zardad AZ, Choonara YE, Claire du Toit L, Kumar P, Mabrouk M, Kondiah PPD, et al. A review of thermo- and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polym. 2016;8(10):359.
Wu CH, Sun MK, Shieh J, Chen CH, Huang CW, Dai CA, et al. Ultrasound-responsive NIPAM-based hydrogels with tunable profile of controlled release of large molecules. Ultrasonics. 2018;83:157–63.
Audebrand M, Kolb M, Axelos MAV. Combined rheological and ultrasonic study of alginate and pectin gels near the sol-gel transition. Biomacromolecules. 2006;7:2811–7.
Jiang H, Kobayashi T. Ultrasound stimulated release of gallic acid from chitin hydrogel matrix. Mater Sci Eng C. 2017;75:478–86.
Lu ZR, Kopeckova P, Kopecek J. Antigen responsive hydrogels based on polymerizable antibody Fab' fragment. Macromol Biosci. 2003;3(6):296–300.
Souza SF, Kogikoski S Jr, Silva ER, Alves WA. Nanostructured antigen responsive hydrogels based on peptides for leishmaniasis detection. J Braz Chem Soc. 2017;28(9):1619–29.
Zhang R, Bowyer A, Eisenthal R, Hubble J. A smart membrane based on an antigen-responsive hydrogel. Biotechnol Bioeng. 2007;97(4):976–84.
Borges O, Borchard G, Verhoef JC, De Sousa A, Junginger HE. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int J Pharm. 2005;299:155–66.
Li XY, Kong XY, Shi S, Zheng XL, Guo G, Wei YQ, et al. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008;8:89.
Thornton PD, McConnel G, Ulijin RV. Enzyme-responsive polymer hydrogel beads. Chem Commun. 2005;47:5913–5.
Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release. 2014;190:352–70.
Lu Y, Sun W, Gu Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J Control Release. 2014;194:1–19.
Ulijin RV. Enzyme-responsive materials: a new class of smart biomaterials. J Mater Chem. 2006;16:2217–25.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003.
Aimetti AA, Machen AJ, Anseth KS. Poly (ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials. 2009;30(30):6048–54.
Lee SC, Kwon IK, Park K. Hydrogels for delivery of bioactive agents: a historical perspective. Adv Drug Deliv Rev. 2013;65:17–20.
Sadat Ebrahimi MM, Schonherr H. Enzyme-sensing chitosan hydrogels. Langmuir. 2014;30:7842–50.
Wang C, Esker AR. Nanocrystalline chitin thin films. Carbohydr Polym. 2014;102:151–8.
Kaur H, Kumar R, Nagendra Babu J, Mittal S. Advances in arsenic biosensor development—a comprehensive review. Biosens Bioelectron. 2015;63:533–45.
Saha N, Saarai A, Roy N, Kitano T, Saha P. Polymeric biomaterial based hydrogels for biomedical applications. J Biomater Nanobiotechnol. 2011;2:85–90.
Guo B, Glavas L, Albertsson AC. Biodegradable and electrically conducting polymers for biomedical applications. Prog Polym Sci. 2013;38(9):1263–86.
Zaman M, Siddique W, Waheed S, Sarfraz RM, Mahmood A, Qureshi J, et al. Hydrogels, their applications and polymers used for hydrogels: a review. IJBPAS. 2015;4(12):6581–603.
Di Z, Shi Z, Ullah MW, Li S, Yang G. A transparent wound dressing based on bacterial cellulose whisker and poly (2-hydroxyethyl methacrylate). Int J Biol Macromol. 2017;105:638–44.
Singh B, Sharma S, Dhiman A. Acacia gum polysaccharide based hydrogel wound dressings: synthesis, characterization, drug delivery and biomedical properties. Carbohydr Polym. 2017;165:294–303.
Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels. 2017;3(1):6.
Barkhordari S, Yadollahi M. Carboxymethyl cellulose capsulated layered double hydroxides/drug nanohybrids for cephalexin oral delivery. Appl Clay Sci. 2016;121-122:77–85.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007.
Masteiková R, Chalupová Z, Šklubalová Z. Stimuli-sensitive hydrogels in controlled and sustained drug delivery. Medicina. 2003;39:19–24.
He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127:189–207.
Kuhn W, Hargitay B, Katchalsky A, Eisenberg H. Reversible dilation and contraction by changing the state of ionization of high-polymer acid networks. Nature. 1950;165:514–6.
Aranaz I, Mengíbar M, Harris R, Paños I, Miralles B, Acosta N, et al. Functional characterization of chitin and chitosan. Curr Chem Biol. 2009;3:203–30.
Kumar A, Han SS. PVA-based hydrogels for tissue engineering: a review. Int J Polym Mater Polym Biomater. 2017;66(4):159–82.
Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–30.
Asadi N, Alizadeh E, Salehi R, Khalandi B, Davaran S, Akbarzadeh A. Nanocomposite hydrogels for cartilage tissue engineering: a review. Artif Cell Nanomed Biotechnol. 2018;46(3):465–71.
Sun J, Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013;6:1285–309.
Gauvin R, Parenteau-Bareil R, Dokmeci MR, Merryman WD, Khademhosseini A. Hydrogels and microtechnologies for engineering the cellular microenvironment. Wires Nanomed Nanobiotechnol. 2012;4:235–46.
Lu Z, Gao J, He Q, Wu J, Liang D, Yang H, et al. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym. 2017;156:460–9.
Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–4.
Qu X, Wirsén A, Albertsson AC. Novel pH-sensitive chitosan hydrogels: swelling behavior and states of water. Polymer. 2000;41(13):4841–7.
Aguirre CI, Reguera E, Stein A. Tunable colors in opals and inverse opal photonic crystals. Adv Funct Mater. 2010;20:2565–78.
Xia M, Cheng Y, Meng Z, Jiang X, Chen Z, Theato P, et al. A novel nanocomposite hydrogel with precisely tunable UCST and LCST. Macromol Rapid Commun. 2015;36(5):477–82.
Hebeish A, Farag S, Sharaf S, Shaheen TI. Thermal responsive hydrogels based on semi interpenetrating network of poly (NIPAm) and cellulose nanowhiskers. Carbohydr Polym. 2014;102:159–66.
Nucara L, Piazza V, Greco F, Robbiano V, Cappello V, Gemmi M, et al. Ionic strength responsive sulfonated polystyrene opals. ACS Appl Mater Interfaces. 2017;9(5):4818–27.
Chen JK, Chang CJ. Fabrications and applications of stimulus-responsive polymer films and patter ns on surfaces: a review. Materials. 2014;7:805–75.
Qiu X, Hu S. Smart materials based on cellulose: a review of the preparations, properties, and applications. Materials. 2013;6:738–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: a Review. Regen. Eng. Transl. Med. 7, 91–114 (2021). https://doi.org/10.1007/s40883-019-00134-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00134-1