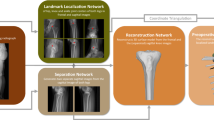
Abstract
Purpose
For the purpose of arthrosis structure strengthening, the reconstructed bone and cartilage are utilized to carry out holistic finite element analysis (FEA), as a whole rather than separated components in order to evaluate the biomechanical performance by convergent validation. This paper presents a biomechanical strengthening design method for human limb articulation based on reconstructed skeleton kinesthetics (RSK).
Methods
The 3D reconstruction of manifold structure including hard bone, medullary cavity and cartilage can be sequentially and progressively implemented from heterogeneous medical images, such as computed tomography (CT) and magnetic resonance imaging (MRI). The sliced images can be recognized to extract boundary contour using deep learning-based intelligent edge detection and classification method. Once the 3D skeleton models are generated, the anatomical coordinate systems, together with joint coordinate system can be created therefrom. Based on Hertz’s contact theory, the methodology of human articulation kinesthetics can be employed to evaluate the flexion mobility.
Results
The equivalent stress, maximum principal stress and material strain energy under diverse loadings can be reckoned as more accurate results via Neo-Hookean hyperelastic model to evaluate biomechanical effect. As a consequence, the limb articulation can be optimized with better biodynamics performance, resulting from better arthrosis structure and its fabrication process thereafter via 3D printing (3DP) or additive manufacturing (AM).
Conclusion
The proposed RSK method can generate individual 3D skeleton articulation in accordance with medical images. Moreover, the RSK can forwardly accomplish biomechanical strengthening design from rehabilitation demands to the kinesthetics performance.










Similar content being viewed by others

References
Schad, P., Wollenweber, M., Thüring, J., Schock, J., Eschweiler, J., Palm, G., Radermacher, K., Eckstein, F., Prescher, A., Kuhl, C., Truhn, D., & Nebelung, S. (2020). Magnetic resonance imaging of human knee joint functionality under variable compressive in-situ loading and axis alignment. Journal of the Mechanical Behavior of Biomedical Materials, 110, 103890. https://doi.org/10.1016/j.jmbbm.2020.103890.
Niedernhuber, M., Barone, D. G., & Lenggenhager, B. (2018). Prostheses as extensions of the body: Progress and challenges. Neuroscience & Biobehavioral Reviews, 92, 1–6. https://doi.org/10.1016/j.neubiorev.2018.04.020.
Jeon, O. H., Kim, C., Laberge, R., Demaria, M., Rathod, S., Vasserot, A. P., Chung, J. W., Kim, D. H., Poon, Y., David, N., Baker, D. J., van Deursen, J. M., Campisi, J., & Elisseeff, J. H. (2017). Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine, 23(6), 775–781. https://doi.org/10.1038/nm.4324.
Aimoto, K., Ota, S., Hase, K., Sakai, T., Kodama, K., & Nakamura, H. (2020). Development of an impulse response method for assessing knee osteoarthritis at the femorotibial joint: Comparison between healthy young adults and older women with clinical knee osteoarthritis. Journal of Medical and Biological Engineering, 40(1), 35–40. https://doi.org/10.1007/s40846-019-00484-9.
Shekarforoush, M., Barton, K. I., Atarod, M., Heard, B. J., Sevick, J. L., Martin, R., Hart, D. A., Frank, C. B., & Shrive, N. G. (2018). An explicit method for analysis of three-dimensional linear and angular velocity of a joint, with specific application to the knee joint. Journal of Medical and Biological Engineering, 38(2), 273–283. https://doi.org/10.1007/s40846-017-0298-1.
Sharma, L., Song, J., Felson, D. T., Cahue, S., Shamiyeh, E., & Dunlop, D. D. (2001). The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA, 286(2), 188–195. https://doi.org/10.1001/jama.286.2.188.
Miranda, D. L., Rainbow, M. J., Leventhal, E. L., Crisco, J. J., & Fleming, B. C. (2010). Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. Journal of Biomechanics, 43(8), 1623–1626. https://doi.org/10.1016/j.jbiomech.2010.01.036.
Kai, S., Sato, T., Koga, Y., Omori, G., Kobayashi, K., Sakamoto, M., & Tanabe, Y. (2014). Automatic construction of an anatomical coordinate system for three-dimensional bone models of the lower extremities—pelvis, femur, and tibia. Journal of Biomechanics, 47(5), 1229–1233. https://doi.org/10.1016/j.jbiomech.2013.12.013.
Kadleček, P., Ichim, A., Liu, T., Křivánek, J., & Kavan, L. (2016). Reconstructing personalized anatomical models for physics-based body animation. Acm Transactions On Graphics, 35(6), 1–13. https://doi.org/10.1145/2980179.2982438.
Theodore, W., Twiggs, J., Kolos, E., Roe, J., Fritsch, B., Dickison, D., Liu, D., Salmon, L., Miles, B., & Howell, S. (2017). Variability in static alignment and kinematics for kinematically aligned TKA. The Knee, 24(4), 733–744. https://doi.org/10.1016/j.knee.2017.04.002.
van Duren, B. H., Pandit, H., Pechon, P., Hart, A., & Murray, D. W. (2018). The role of the patellar tendon angle and patellar flexion angle in the interpretation of sagittal plane kinematics of the knee after knee arthroplasty: A modelling analysis. The Knee, 25(2), 240–248. https://doi.org/10.1016/j.knee.2018.01.006.
Jakus, A. E., Rutz, A. L., Jordan, S. W., Kannan, A., Mitchell, S. M., Yun, C., Koube, K. D., Yoo, S. C., Whiteley, H. E., Richter, C., Galiano, R. D., Hsu, W. K., Stock, S. R., Hsu, E. L., & Shah, R. N. (2016). Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Science Translational Medicine, 8(358), 127r–358r. https://doi.org/10.1126/scitranslmed.aaf7704.
Yan, L., Lim, J. L., Lee, J. W., Tia, C. S. H., O’Neill, G. K., & Chong, D. Y. R. (2020). Finite element analysis of bone and implant stresses for customized 3D-printed orthopaedic implants in fracture fixation. Medical & Biological Engineering & Computing, 58, 921–931. https://doi.org/10.1007/s11517-019-02104-9.
Sekiguchi, Y., Kokubun, T., Hanawa, H., Shono, H., Tsuruta, A., & Kanemura, N. (2020). Foot kinematics of impact absorption and force exertion during depth-jump using a multi-segment foot model. Journal of Medical and Biological Engineering, 40(5), 757–765. https://doi.org/10.1007/s40846-020-00560-5.
Pejhan, S., Khosravipour, I., Gascoyne, T., Bohm, E., Brandt, J., Luo, Y., & Wyss, U. (2019). Evaluation of the tibiofemoral contact characteristics of a customized surface-guided knee implant. Journal of Medical and Biological Engineering, 39(2), 205–212. https://doi.org/10.1007/s40846-018-0399-5.
Thienkarochanakul, K., Javadi, A. A., Akrami, M., Charnley, J. R., & Benattayallah, A. (2020). Stress distribution of the tibiofemoral joint in a healthy versus osteoarthritis knee model using image-based three-dimensional finite element analysis. Journal of Medical and Biological Engineering, 40(3), 409–418. https://doi.org/10.1007/s40846-020-00523-w.
Chen, Y., Xie, Z., Huang, K., Lin, H., & Chang, J. (2020). Biomechanical analysis to determine the most effective posture during squats and shallow squats while lifting weights in women. Journal of Medical and Biological Engineering, 40(3), 334–339. https://doi.org/10.1007/s40846-020-00513-y.
Sato, T., Mochizuki, T., Katsumi, R., & Takahashi, Y. (2020). Functionally oriented alignment of the lower extremity reflecting the direction of gait for healthy elderly, knee osteoarthritis, and total knee arthroplasty subjects. Journal of Medical and Biological Engineering, 40(6), 887–898. https://doi.org/10.1007/s40846-020-00569-w.
Yang, Y., Yang, G., Song, Y., Xu, Y., Zhao, S., & Zhang, W. (2020). 3D bioprinted integrated osteochondral scaffold-mediated repair of articular cartilage defects in the rabbit knee. Journal of Medical and Biological Engineering, 40(1), 71–81. https://doi.org/10.1007/s40846-019-00481-y.
Boyle, C., & Kim, I. Y. (2011). Comparison of different hip prosthesis shapes considering micro-level bone remodeling and stress-shielding criteria using three-dimensional design space topology optimization. Journal of Biomechanics, 44(9), 1722–1728. https://doi.org/10.1016/j.jbiomech.2011.03.038.
Park, J., Sutradhar, A., Shah, J. J., & Paulino, G. H. (2018). Design of complex bone internal structure using topology optimization with perimeter control. Computers in Biology and Medicine, 94, 74–84. https://doi.org/10.1016/j.compbiomed.2018.01.001.
Goda, I., Ganghoffer, J., Czarnecki, S., Czubacki, R., & Wawruch, P. (2019). Topology optimization of bone using cubic material design and evolutionary methods based on internal remodeling. Mechanics Research Communications, 95, 52–60. https://doi.org/10.1016/j.mechrescom.2018.12.003.
Peto, M., Ramírez-Cedillo, E., Hernández, A., & Siller, H. R. (2019). Structural design optimization of knee replacement implants for additive manufacturing. Procedia Manufacturing, 34, 574–583. https://doi.org/10.1016/j.promfg.2019.06.222.
Xu, J., Wang, K., Gao, M., Tu, Z., & Tan, J. (2020). Biomechanical performance design of joint prosthesis for medical rehabilitation via generative structure optimization. Computer Methods in Biomechanics and Biomedical Engineering, 11, 1–17. https://doi.org/10.1080/10255842.2020.1789970.
Xu, J., Tao, M., Zhang, S., Jiang, X., & Tan, J. (2021). Non-rigid registration of biomedical image for radiotherapy based on adaptive feature density flow. Biomedical Signal Processing and Control, 68, 102691. https://doi.org/10.1016/j.bspc.2021.102691.
Xu, J., Gao, M., Feng, X., Su, Z., Wang, K., Zhang, S., & Tan, J. (2021). Support diminution design for layered manufacturing of manifold surface based on variable orientation tracking. 3D Printing and Additive Manufacturing, 8(3), 149–167. https://doi.org/10.1089/3dp.2020.0203.
Grood, E. S., & Suntay, W. J. (1983). A joint coordinate system for the clinical description of three- dimensional motions: To the knee. Journal of Biomechanical Engineering, 105(2), 136–144. https://doi.org/10.1115/1.3138397.
Penrose, J. M., Holt, G. M., Beaugonin, M., & Hose, D. R. (2002). Development of an accurate three-dimensional finite element knee model. Computer Methods in Biomechanics and Biomedical Engineering, 5(4), 291–300. https://doi.org/10.1080/1025584021000009724.
Louis, M. J., & Malayalamurthi, R. (2017). Stress response of patellofemoral joint subjected to femoral retroversion with various patellar kinematics and flexions—an FEA study. Biocybernetics and Biomedical Engineering, 37(2), 316–325. https://doi.org/10.1016/j.bbe.2016.12.006.
Dai, Y., Scuderi, G. R., Penninger, C., Bischoff, J. E., & Rosenberg, A. (2014). Increased shape and size offerings of femoral components improve fit during total knee arthroplasty. Knee Surgery Sports Traumatology Arthroscopy, 22(12), 2931–2940. https://doi.org/10.1007/s00167-014-3163-6.
Dai, Y., Scuderi, G. R., Bischoff, J. E., Bertin, K., Tarabichi, S., & Rajgopal, A. (2014). Anatomic tibial component design can increase tibial coverage and rotational alignment accuracy: A comparison of six contemporary designs. Knee Surgery Sports Traumatology Arthroscopy, 22(12), 2911–2923. https://doi.org/10.1007/s00167-014-3282-0.
Sheng, W., Ji, A., & Chen, C. (2017). Biomechanical research of the femur finite element model combined with different material assignment methods. MATEC Web of Conferences, 104, 2022. https://doi.org/10.1051/matecconf/201710402022.
Gatenholm, B., Lindahl, C., Brittberg, M., & Stadelmann, V. A. (2019). Spatially matching morphometric assessment of cartilage and subchondral bone in osteoarthritic human knee joint with micro-computed tomography. Bone, 120, 393–402. https://doi.org/10.1016/j.bone.2018.12.003.
Gokkus, K., Atmaca, H., Uğur, L., Özkan, A., & Aydin, A. T. (2016). The relationship between medial meniscal subluxation and stress distribution pattern of the knee joint: Finite element analysis. Journal of orthopaedic science Official Journal of the Japanese Orthopaedic Association, 21(1), 32–37. https://doi.org/10.1016/j.jos.2015.10.001.
Zhang, K., Li, L., Yang, L., Shi, J., Zhu, L., Liang, H., Wang, X., Yang, X., & Jiang, Q. (2019). The biomechanical changes of load distribution with l21ongitudinal tears of meniscal horns on knee joint: a finite element analysis. Journal of Orthopaedic Surgery and Research, 14, 237–248. https://doi.org/10.1186/s13018-019-1255-1.
Acknowledgements
The work presented in this article is funded by the National Natural Science Foundation of China (51775494; 51935009; 51821093), Zhejiang University president special fund financed by Zhejiang province (No. K20210255), Zhejiang provincial key research and development project of China (2019C01141; LGG21E050020).
Author information
Authors and Affiliations
Contributions
JX established the biomechanical kinesthetics via computer science and finalized the article as first author. ZT carried out the FEM calculations to draft the manuscript. JX evaluated the biomedical effectiveness via clinical practice. SZ guided the team research as principle investigator (PI). JT made constructive suggestions to the team research as chief scientist.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this work involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Xu, JH., Tu, ZX., Xu, JX. et al. Biomechanical Strengthening Design for Limb Articulation Based on Reconstructed Skeleton Kinesthetics. J. Med. Biol. Eng. 41, 715–729 (2021). https://doi.org/10.1007/s40846-021-00645-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-021-00645-9