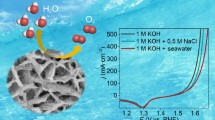
Abstract
The development of efficient electrocatalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) with excellent catalytic performance and stability plays key roles in the commercialization of water splitting to generate hydrogen energy. Herein, a 2D-3D nanostructure composed of metal hydroxides and Prussian blue analogus (PBA) was in-situ decorated onto the NiFe foam (Pt-NiFe PBA) through a facile and scalable corrosive-coordinate approach. The specifically designed morphology favored the provision of abundant active sites, optimized the reaction pathway, and accelerated mass transport during the electrocatalytic process. Consequently, the as-synthesized Pt-NiFe PBA reached 10 mA cm−2 with small overpotentials of 29 and 210 mV in 1 mol L−1 KOH deionized water for HER and OER, respectively. Remarkably, Pt-NiFe PBA required an overpotential of 21 mV to drive 10 mA cm−2 in seawater containing 1 mol L−1 KOH with prominent durability. Moreover, with the as-synthesized Pt-NiFe PBA as bifunctional electrocatalyst, the Pt-NiFe PBA∥Pt-NiFe PBA electrolyzer needed 1.46 and 1.48 V to drive 10 mA cm−2 in 1 mol L−1 KOH with deionized water and 1 mol L−1 KOH with seawater, respectively. Remarkably, sustainable energies were utilized to power the overall water splitting and stored as easily portable hydrogen energy.
摘要
开发具有良好的催化性能和稳定性的析氢反应(HER)和析氧反 应(OER)的电催化剂对水分解产氢的商业化起着关键作用. 本文通过简 单、可扩展的腐蚀配位方法, 将金属氢氧化物和金属有机骨架(MOF) 组成的二维-三维(2D-3D)纳米结构原位装饰在泡沫NiFe (Pt-NiFe PBA)上. 所设计的特殊形态有利于在电催化过程中提供丰富的活性位 点、优化反应途径和加速传质. 因此, 合成的Pt-NiFe PBA在1 mol L−1 KOH中HER和OER在10 mA cm−2时具有29和210 mV的过电位. 值得注 意的是, 在10 mA cm−2 时该催化剂仅需21 mV即可驱动1 mol L−1 KOH 海水, 并具有出色的稳定性. 此外, 将合成的Pt-NiFe PBA用作双功能电 催化剂时, 只需1.46和1.48 V即可以达到10 mA cm−2. 此外, 间歇性的可 持续能源, 如热能、风能和太阳能可以为该水分解器提供动力.
Similar content being viewed by others
References
Jing H, Zhu P, Zheng X, et al. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv Powder Mater, 2021, doi: https://doi.org/10.1016/j.apmate.2021.10.004
Fang W, Huang L, Zaman S, et al. Recent progress on two-dimensional electrocatalysis. Chem Res Chin Univ, 2020, 36: 611–621
Liu Y, Feng Q, Liu W, et al. Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification. Nano Energy, 2021, 81: 105641
Gong L, Yang H, Wang H, et al. Corrosion formation and phase transformation of nickel-iron hydroxide nanosheets array for efficient water oxidation. Nano Res, 2021, 14: 4528–4533
Pan Y, Zhang C, Lin Y, et al. Electrocatalyst engineering and structure-activity relationship in hydrogen evolution reaction: From nanostructures to single atoms. Sci China Mater, 2020, 63: 921–948
Wang TJ, Sun HY, Xue Q, et al. Holey platinum nanotubes for ethanol electrochemical reforming in aqueous solution. Sci Bull, 2021, 66: 2079–2089
Shan J, Ye C, Chen S, et al. Short-range ordered iridium single atoms integrated into cobalt oxide spinel structure for highly efficient electrocatalytic water oxidation. J Am Chem Soc, 2021, 143: 5201–5211
Zheng F, Zhang W, Zhang X, et al. Sub-2 nm ultrathin and robust 2D FeNi layered double hydroxide nanosheets packed with 1D FeNi-MOFs for enhanced oxygen evolution electrocatalysis. Adv Funct Mater, 2021, 31: 2103318
Yu W, Gao Y, Chen Z, et al. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chin J Catal, 2021, 42: 1876–1902
Benck JD, Hellstern TR, Kibsgaard J, et al. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal, 2014, 4: 3957–3971
Mahmood J, Li F, Jung SM, et al. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat Nanotech, 2017, 12: 441–446
Popczun EJ, Read CG, Roske CW, et al. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew Chem Int Ed, 2014, 53: 5427–5430
Zhou W, Wu M, Li G. Rambutan-like CoP@Mo-Co-O hollow microspheres for efficient hydrogen evolution reaction in alkaline solution. Chin J Catal, 2020, 41: 691–697
Jin H, Gu Q, Chen B, et al. Molten salt-directed catalytic synthesis of 2D layered transition-metal nitrides for efficient hydrogen evolution. Chem, 2020, 6: 2382–2394
Yang J, Li W, Wang D, et al. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv Mater, 2020, 32: 2003300
Chen Q, Nie Y, Ming M, et al. Sustainable synthesis of supported metal nanocatalysts for electrochemical hydrogen evolution. Chin J Catal, 2020, 41: 1791–1811
Wu Z, Zhao Y, Wu H, et al. Corrosion engineering on iron foam toward efficiently electrocatalytic overall water splitting powered by sustainable energy. Adv Funct Mater, 2021, 31: 2010437
Yu X, Chen G, Wang Y, et al. Hierarchical coupling effect in hollow Ni/NiFe2O4-CNTs microsphere via spray-drying for enhanced oxygen evolution electrocatalysis. Nano Res, 2020, 13: 437–446
Xue Q, Bai XY, Zhao Y, et al. Au core-PtAu alloy shell nanowires for formic acid electrolysis. J Energy Chem, 2022, 65: 94–102
Wang J, Zhang Z, Song H, et al. Water dissociation kinetic-oriented design of nickel sulfides via tailored dual sites for efficient alkaline hydrogen evolution. Adv Funct Mater, 2021, 31: 2008578
Zhang X, Zhao Y, Zhao Y, et al. A simple synthetic strategy toward defect-rich porous monolayer NiFe-layered double hydroxide nanosheets for efficient electrocatalytic water oxidation. Adv Energy Mater, 2019, 9: 1900881
Song M, Zhang Z, Li Q, et al. Ni-foam supported Co(OH)F and Co-P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J Mater Chem A, 2019, 7: 3697–3703
Cong M, Sun D, Zhang L, et al. In situ assembly of metal-organic framework-derived N-doped carbon/Co/CoP catalysts on carbon paper for water splitting in alkaline electrolytes. Chin J Catal, 2020, 41: 242–248
Smialkowski M, Tetzlaff D, Hensgen L, et al. Fe/Co and Ni/Co-pentlandite type electrocatalysts for the hydrogen evolution reaction. Chin J Catal, 2021, 42: 1360–1369
Mao J, He CT, Pei J, et al. Isolated Ni atoms dispersed on Ru nanosheets: High-performance electrocatalysts toward hydrogen oxidation reaction. Nano Lett, 2020, 20: 3442–3448
Yuan Z, Bak SM, Li P, et al. Activating layered double hydroxide with multivacancies by memory effect for energy-efficient hydrogen production at neutral pH. ACS Energy Lett, 2019, 4: 1412–1418
Huang Y, Li M, Yang W, et al. 3D ordered mesoporous cobalt ferrite phosphides for overall water splitting. Sci China Mater, 2020, 63: 240–248
Meng X, Ma C, Jiang L, et al. Distance synergy of MoS2-confined rhodium atoms for highly efficient hydrogen evolution. Angew Chem, 2020, 132: 10588–10593
Zhang L, Xiao W, Zhang Y, et al. Nanocarbon encapsulating Ni-doped MoP/graphene composites for highly improved electrocatalytic hydrogen evolution reaction. Compos Commun, 2021, 26: 100792
Li P, Duan X, Kuang Y, et al. Tuning electronic structure of NiFe layered double hydroxides with vanadium doping toward high efficient electrocatalytic water oxidation. Adv Energy Mater, 2018, 8: 1703341
Lu F, Yi D, Liu S, et al. Engineering platinum-oxygen dual catalytic sites via charge transfer towards highly efficient hydrogen evolution. Angew Chem, 2020, 132: 17865–17871
Wang H, Zhang X, Wang J, et al. Puffing quaternary FexCoxNi1−x−yP nanoarray via kinetically controlled alkaline etching for robust overall water splitting. Sci China Mater, 2020, 63: 1054–1064
Lv L, Yang Z, Chen K, et al. 2D layered double hydroxides for oxygen evolution reaction: From fundamental design to application. Adv Energy Mater, 2019, 9: 1803358
Zhang J, Yu L, Chen Y, et al. Designed formation of double-shelled Ni-Fe layered-double-hydroxide nanocages for efficient oxygen evolution reaction. Adv Mater, 2020, 32: 1906432
Bo X, Dastafkan K, Zhao C. Design of multi-metallic-based electrocatalysts for enhanced water oxidation. ChemPhysChem, 2019, 20: 2936–2945
Zhai P, Zhang Y, Wu Y, et al. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat Commun, 2020, 11: 5462
Sun F, Wang G, Ding Y, et al. NiFe-based metal-organic framework nanosheets directly supported on nickel foam acting as robust electrodes for electrochemical oxygen evolution reaction. Adv Energy Mater, 2018, 8: 1800584
Li Z, Wu X, Jiang X, et al. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv Powder Mater, 2021, doi: https://doi.org/10.1016/j.apmate.2021.11.007
Wang Y, Yan L, Dastafkan K, et al. Lattice matching growth of conductive hierarchical porous MOF/LDH heteronanotube arrays for highly efficient water oxidation. Adv Mater, 2021, 33: 2006351
Zhang Z, Li X, Zhong C, et al. Spontaneous synthesis of silver-nanoparticle-decorated transition-metal hydroxides for enhanced oxygen evolution reaction. Angew Chem Int Ed, 2020, 59: 7245–7250
Luo J, Wang XH, Shen L, et al. Corrosion-engineered Mo-containing FeCo-(oxy)hydroxide electrocatalysts for superior oxygen evolution reaction. ACS Sustain Chem Eng, 2021, 9: 12233–12241
Xuan C, Peng Z, Xia K, et al. Self-supported ternary Ni-Fe-P nanosheets derived from metal-organic frameworks as efficient overall water splitting electrocatalysts. Electrochim Acta, 2017, 258: 423–432
Yang H, Wang C, Zhang Y, et al. Green synthesis of NiFe LDH/Ni foam at room temperature for highly efficient electrocatalytic oxygen evolution reaction. Sci China Mater, 2019, 62: 681–689
Ma Y, Wang B, Wang Q, et al. Facile synthesis of α-FeOOH/γ-Fe2O3 by a pH gradient method and the role of γ-Fe2O3 in H2O2 activation under visible light irradiation. Chem Eng J, 2018, 354: 75–84
Zhang G, Li Y, Xiao X, et al. In situ anchoring polymetallic phosphide nanoparticles within porous Prussian blue analogue nanocages for boosting oxygen evolution catalysis. Nano Lett, 2021, 21: 3016–3025
Wu Z, Guo X, Zhang Z, et al. Interface engineering of MoS2 for electrocatalytic performance optimization for hydrogen generation via urea electrolysis. ACS Sustain Chem Eng, 2019, 7: 16577–16584
Hao S, Chen L, Yu C, et al. NiCoMo hydroxide nanosheet arrays synthesized via chloride corrosion for overall water splitting. ACS Energy Lett, 2019, 4: 952–959
Liu X, Guo X, Gong M, et al. Corrosion-assisted large-scale production of hierarchical iron rusts/Ni(OH)2 nanosheet-on-microsphere arrays for efficient electrocatalysis. Electrochim Acta, 2020, 353: 136478
Wang K, Du H, He S, et al. Kinetically controlled, scalable synthesis of γ-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated γ-NiOOH. Adv Mater, 2021, 33: 2005587
Song P, Feng JJ, Zhong SX, et al. Facile preparation of reduced graphene oxide supported PtNi alloyed nanosnowflakes with high catalytic activity. RSC Adv, 2015, 5: 35551–35557
Gao ZD, Han YY, Li YC, et al. Photocatalytic synthesis and synergistic effect of Prussian blue-decorated Au nanoparticles/TiO2 nanotube arrays for H2O2 amperometric sensing. Electrochim Acta, 2014, 125: 530–535
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (22002068, 51772162 and 52072197), the Youth Innovation and Technology Foundation of Shandong Higher Education Institutions, China (2019KJC004), the Outstanding Youth Foundation of Shandong Province (ZR2019JQ14), Taishan Scholar Young Talent Program (tsqn201909114), the Major Scientific and Technological Innovation Project (2019JZZY020405), the Major Basic Research Program of Natural Science Foundation of Shandong Province (ZR2020ZD09), China Postdoctoral Science Foundation (2021M691700), the Natural Science Foundation of Shandong Province of China (ZR2019BB002, ZR2018BB031), Australian Research Future Fellowship (FT210100298), CSIRO Energy Centre, and the Victorian Government’s support through the provision of a grant from Veski-Study Melbourne Research Partnerships Project.
Author information
Authors and Affiliations
Contributions
Chen Z performed the experiments and wrote the paper with support from Wu Z and Wang L. All authors contributed to the general discussion.
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Experimental details and supporting data are available in the online version of the paper.
Zhi Chen graduated from Zaozhuang University in 2019. He is currently studying at the School of Chemistry and Molecular Engineering, Qingdao University of Science and Technology (QUST), under Dr. Zexing Wu. His current research interest is the design and synthesis of high-efficiency electrocatalysts for overall water splitting.
Lei Wang received his PhD degree in 2006 from Jilin University. Afterward, he joined the Faculty of QUST, where he was the deputy director of the Key Laboratory of Eco-Chemical Engineering. Now he is the director of the College of Environment and Safety Engineering. From 2008 to 2010, he worked as a postdoctoral fellow in the State Key Laboratory of Crystal Materials, Shandong University. His research interests currently focus on the design and synthesis of porous MOF materials, functional inorganic materials, and their applications in gas separation, photocatalysis, lithium-ion battery, etc.
Zexing Wu is currently working at the School of Chemistry and Molecular Engineering, QUST. He received his BSc from Binzhou University, and PhD from Huazhong University of Science & Technology. His research mainly focuses on the design and fabrication of nanomaterials with high performance in energy storage and conversion devices.
Supporting Information
40843_2021_1943_MOESM1_ESM.pdf
Corrosive-Coordinate Engineering to Construct 2D-3D Nanostructure with Trace Pt as Efficient Bifunctional Electrocatalyst for Overall Water-splitting
Rights and permissions
About this article
Cite this article
Chen, Z., Liu, D., Gao, Y. et al. Corrosive-coordinate engineering to construct 2D-3D nanostructure with trace Pt as efficient bifunctional electrocatalyst for overall water splitting. Sci. China Mater. 65, 1217–1224 (2022). https://doi.org/10.1007/s40843-021-1943-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1943-5