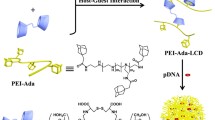
Abstract
Incorporating functional ligands and biodegradable bonds into biocompatible low-molecular-weight (LMW) polymers, such as 1.8 kDa poly(ethylenimine) (PEI 1.8k), is a common strategy to improve the properties of LMW polymers including biosafety and delivery efficacy. This study demonstrates the hypothesis that introducing different functional ligands and linked reductive disulfides in PEI 1.8k will achieve superior siRNA transfection efficiency. By incorporating PEI-X (X represents cholesterol (Ch), heptafluorobutyric anhydride (HFBA, F) and 4-carboxyphenylboronic acid (PBA)) functional ligands into PEI 1.8k and subsequently crosslinking with each other via disulfide bond links, reductive-responsive PEI-X-SS-X-PEI copolymers were constructed to enhance the cellular transfection via the synergistic effect of the high affinity of Ch, F and PBA to cell membranes and the disulfide reduction triggered intracellular disassembly of micelles and subsequent siRNA release. Extraordinarily, ternary Ch-SS-F-SS-PBA micelles exhibited the strongest siRNA transfection efficiencies in in vitro cell experiments and in vivo animal experiments due to the coordination of enhanced serum stability, promoted cell uptake and endosomal escape, and cell targeting ability. This strategy of constructed multifunctional polymer here we called “building-block crosslinking” showed a simple and smart way to synthesize new materials. Also this strategy of constructing ligands-directed reduction-sensitive micelles improves the transfection efficiency of LMW PEI and provides a valuable insight to develop novel gene delivery systems.
摘要
将功能性配体和生物可降解性键结合到生物相容性的低分子量(LMW)聚合物中, 如1.8k聚乙烯亚胺(PEI), 是提高LMW聚合物生物安全性和递送效率的常见策略. 本研究证明了在PEI 1.8k中引入不同功能性配体并使用还原性二硫键将其交联可获得超高siRNA递送效率. 通过将X (X代表胆固醇(Ch)、七氟丁酸酐(HFBA, F)和4-羧苯基硼酸(PBA))功能配体分别引入PEI 1.8k中得到PEI-X单体聚合物, 然后将三者通过二硫键相互交联可成功构建还原响应性PEI-X-SS-X-PEI共聚物. 利用Ch、 F、 PBA对细胞膜的高亲和力、 还原性二硫键触发的细胞内胶束解体及随后siRNA释放的协同作用, PEI-X-SS-X-PEI可显著增强siRNA的细胞转染. 这种三元型Ch-SS-F-SS-PBA/siRNA形成的胶束在体外细胞实验和体内动物实验中均表现出最强的siRNA转染效率, 这得益于胶束具有增强的血清稳定性、 提升的细胞摄取和溶酶体逃逸能力以及细胞靶向性的协同作用. 这种“嵌段交联”型的多功能聚合物的构建展示了一种简单并智能的材料合成方法. 同时, 这种配体介导构建还原敏感型胶束的策略提高了LMW-PEI的转染效率, 为开发新的基因递送系统提供了有价值的思路.
Similar content being viewed by others
References
Scholz C, Wagner E. Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J Control Release, 2012, 161: 554–565
Zhu HY, Zhang SY, Ling Y, et al. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J Control Release, 2015, 220: 529–544
Dong Y, Siegwart DJ, Anderson DG. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliver Rev, 2019, 144: 133–147
Singhsa P, Diaz-Dussan D, Manuspiya H, et al. Well-defined cationic N-[3-(dimethylamino)propyl]methacrylamide hydrochloride-based (co)polymers for siRNA delivery. Biomacromolecules, 2018, 19: 209–221
Saw PE, Yao H, Lin C, et al. Stimuli-responsive polymer-prodrug hybrid nanoplatform for multistage siRNA delivery and combination cancer therapy. Nano Lett, 2019, 19: 5967–5974
Shen W, Wang Q, Shen Y, et al. Green tea catechin dramatically promotes RNAi mediated by low-molecular-weight polymers. ACS Cent Sci, 2018, 4: 1326–1333
Fan QQ, Zhang CL, Qiao JB, et al. Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials, 2020, 230: 119616
Oupický D, Li J. Bioreducible polycations in nucleic acid delivery: past, present, and future trends. Macromol Biosci, 2014, 14: 908–922
Zhao Y, Zhao W, Lim YC, et al. Salinomycin-loaded gold nanoparticles for treating cancer stem cells by ferroptosis-induced cell death. Mol Pharm, 2019, 16: 2532–2539
Wang H, Chang H, Zhang Q, et al. Fabrication of low-generation dendrimers into nanostructures for efficient and nontoxic gene delivery. Top Curr Chem (Z), 2017, 375: 62
Wang F, Chen L, Zhang R, et al. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J Control Release, 2014, 196: 222–233
Luo J, Wagner E, Wang Y. Artificial peptides for antitumoral siRNA delivery. J Mater Chem B, 2020, 8: 2020–2031
Oliveira ACN, Fernandes J, Gonçalves A, et al. Lipid-based nanocarriers for siRNA delivery: challenges, strategies and the lessons learned from the DODAX: MO liposomal system. Curr Drug Targets, 2019, 20: 29–50
Tai W. Chemical modulation of siRNA lipophilicity for efficient delivery. J Control Release, 2019, 307: 98–107
Werfel TA, Jackson MA, Kavanaugh TE, et al. Combinatorial optimization of PEG architecture and hydrophobic content improves ternary siRNA polyplex stability, pharmacokinetics, and potency in vivo. J Control Release, 2017, 255: 12–26
Chen G, Wang K, Wang Y, et al. Fluorination enhances serum stability of bioreducible poly(amido amine) polyplexes and enables efficient intravenous siRNA delivery. Adv Healthcare Mater, 2018, 7: 1700978
Zhu JY, Zeng X, Qin SY, et al. Acidity-responsive gene delivery for “superfast” nuclear translocation and transfection with high efficiency. Biomaterials, 2016, 83: 79–92
Chen G, Wang K, Hu Q, et al. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl Mater Interfaces, 2017, 9: 4457–4466
Zhang L, Liu F, Li G, et al. Twin-arginine translocation peptide conjugated epirubicin-loaded nanoparticles for enhanced tumor penetrating and targeting. J Pharmaceutical Sci, 2015, 104: 4185–4196
Wang Y, Li J, Chen Y, et al. Balancing polymer hydrophobicity for ligand presentation and siRNA delivery in dual function CXCR4 inhibiting polyplexes. Biomater Sci, 2015, 3: 1114–1123
Xiong SD, Li L, Jiang J, et al. Cationic fluorine-containing amphiphilic graft copolymers as DNA carriers. Biomaterials, 2010, 31: 2673–2685
Wang M, Liu H, Li L, et al. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat Commun, 2014, 5: 3053
Cheng YY. Fluorinated polymers in gene delivery. Acta Polym Sin, 2017: 1234–1245
Peng Q, Chen F, Zhong Z, et al. Enhanced gene transfection capability of polyethylenimine by incorporating boronic acid groups. Chem Commun, 2010, 46: 5888
Li L, Bai Z, Levkin PA. Boronate-dextran: an acid-responsive biodegradable polymer for drug delivery. Biomaterials, 2013, 34: 8504–8510
Liu C, Wan T, Wang H, et al. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv, 2019, 5: eaaw8922
Liu C, Shao N, Wang Y, et al. Clustering small dendrimers into nanoaggregates for efficient DNA and siRNA delivery with minimal toxicity. Adv Healthcare Mater, 2016, 5: 584–592
Lv J, Liu C, Lv K, et al. Boronic acid-rich dendrimer for efficient intracellular peptide delivery. Sci China Mater, 2020, 63: 620–628
Kim J, Lee YM, Kim H, et al. Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials, 2016, 75: 102–111
Chen G, Wang K, Wu P, et al. Development of fluorinated polyplex nanoemulsions for improved small interfering RNA delivery and cancer therapy. Nano Res, 2018, 11: 3746–3761
Yang J, Hendricks W, Liu G, et al. A nanoparticle formulation that selectively transfects metastatic tumors in mice. Proc Natl Acad Sci USA, 2013, 110: 14717–14722
Thomas M, Lu JJ, Ge Q, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA, 2005, 102: 5679–5684
Breunig M, Lungwitz U, Liebl R, et al. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci USA, 2007, 104: 14454–14459
Remant Bahadur KC, Uludağ H. A comparative evaluation of disulfide-linked and hydrophobically-modified PEI for plasmid delivery. J BioMater Sci Polym Ed, 2011, 22: 873–892
Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA, 2010, 107: 3317–3322
Wang M, Cheng Y. Structure-activity relationships of fluorinated dendrimers in DNA and siRNA delivery. Acta Biomater, 2016, 46: 204–210
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81903556), and the Natural Science Fund for Colleges and Universities in Jiangsu Province (19KJB350004). Dr. Liu T was supported by the National Health and Medical Research Council (NHMRC) Early Career Fellowship (1112258) of Australia.
Author information
Authors and Affiliations
Contributions
Author contributions Wu P and Wang K were responsible for the study design; Wang K and Ding D conceived the project and acted as project leader; Wu P and Yin S performed the actuation experiments; Wu P and Yin S were responsible for the manuscript writing; Wu P and Liu T were responsible for the data analyses. All the authors commented and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest> The authors declare that they have no conflict of interest.
Additional information
Pengkai Wu received his PhD degree from China Pharmaceutical University with Prof. Oupický in 2019. He also conducted his work at the University of Nebraska Medical Center as a postdoctoral fellow. His research interests mainly focus on the delivery of drug/siRNA and drug/miRNA combinations in the treatment of liver diseases.
Dan Ding received his PhD degree from Nanjing University in 2010. After a postdoctoral training in the National University of Singapore, he joined Nankai University, where he is currently a professor in the College of Life Sciences. He also conducted his work in The Hong Kong University of Science and Technology as a visiting scholar. His current research focuses on the design and synthesis of smart/functional molecular imaging probes and exploration of their biomedical applications.
Kaikai Wang received his PhD degree in pharmacy with Prof. Yiqiao Hu and Prof. Jinhui Wu from Nanjing University in 2015. He joined China Pharmaceutical University as a postdoc in Prof. Oupicky’s group in 2015. He is now working as an associate professor in Nantong University. His research currently focuses on the development of multifunctional formulations based on novel polymers and proteins for photodynamic and photothermal therapy applications.
Rights and permissions
About this article
Cite this article
Wu, P., Yin, S., Liu, T. et al. “Building-block crosslinking” micelles for enhancing cellular transfection of biocompatible polycations. Sci. China Mater. 64, 241–251 (2021). https://doi.org/10.1007/s40843-020-1366-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1366-2