Abstract
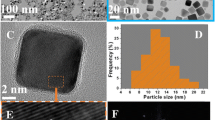
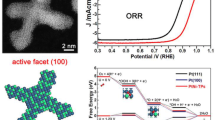
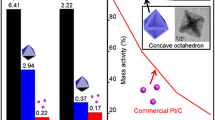
Pt-Ir nanocubes with (100)-terminated facets were synthesized for the first time and their unusual high electrocatalytic activity for a model reaction (i.e., ammonia oxidation) was reported. The key parameters in controlling the shape of the Pt-Ir nanocubes were systematically investigated by transmission electron microscopy (TEM). The electrocatalytic activities of the prepared Pt-Ir and pure Pt nanoparticles (NPs) were characterized by cyclic voltammetry (CV). The results showed that the amount of W(CO)6 and the volume ratio of oleylamine and oleic acid play a significant role in the development of well-defined Pt-Ir nanocubes. The resultant Pt-Ir nanocubes exhibit (100) orientation, which has been confirmed by not only the structural characterization results from high-resolution TEM (HRTEM) and X-ray diffraction (XRD) but also hydrogen desorption profiles obtained from the CV measurements in H2SO4 solution. Lattice contraction of the Pt-Ir nanocubes were suggested by HRTEM and XRD measurements, and the electronic interactions between Pt and Ir in the Pt-Ir nanocubes were demonstrated by X-ray photoelectron spectroscopy. The Pt-Ir nanocubes show higher specific activity than pure Pt nanocubes and much higher specific activity than the polycrystalline Pt-Ir NPs. The much improved specific activity of the Pt-Ir nanocubes could be attributed to the reason that the introduction of Ir in the Pt-Ir nanocubes largely maintains the highly active Pt (100) sites and thus a positive synergistic effect through the addition of Ir to Pt could be achieved due to the possible bifunctional mechanism and the electronic effect.
摘要
同时控制纳米贵金属颗粒的形状(表面原子结构)和成分对进一步提升其性能具有重要意义. 针对铂铱合金纳米颗粒, 已有研究发现铱原子的引入会降低铂基合金纳米颗粒在一些体系中的催化活性. 本文首次制备了具有(100)择优晶面的铂铱立方体纳米颗粒, 并在一(100)敏感的模型反应(即氨的电催化氧化反应)中, 发现其特殊的高催化活性. 所制备的铂铱立方体纳米颗粒具有规则的(100)晶面特征, 并伴随有晶格收缩现象, 此外铂和铱存在电子交互作用. 铂铱立方体纳米颗粒的特定催化活性高于纯铂纳米颗粒, 并远高于普通多晶铂铱纳米颗粒. 该现象一方面可归因于具有高催化活性的铂(100)活性点, 另一方面可归因于铱和铂的协同效应. 以上研究结果表明, 在铂基合金纳米颗粒形状可控的前提下, 引入铱原子可进一步提升其催化活性, 这对于发展具有高催化活性的贵金属纳米颗粒具有一定的指导意义.
Similar content being viewed by others
References
Chen C, Kang Y, Huo Z, et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science, 2014, 343:1339–1343
Liao HB, Hou YL. Liquid-phase templateless synthesis of Pt-on-Pd0.85Bi0.15 nanowires and PtPdBi porous nanoparticles with superior electrocatalytic activity. Chem Mater, 2013, 25:457–465
Liao HB, Zhu JH, Hou YL. Synthesis and electrocatalytic properties of PtBi nanoplatelets and PdBi nanowires. Nanoscale, 2014, 6: 1049–1055
Wu YE, Wang DS, Chen XB, et al. Defect-dominated shape recovery of nanocrystals: a new strategy for trimetallic catalysts. J Am Chem Soc, 2013, 135: 12220–12223
Wu YE, Wang DS, Li YD. Nanocrystals from solutions: catalysts. Chem Soc Rev, 2014, 43: 2112–2124
Wu YE, Wang DS, Zhou G, et al. Sophisticated construction of Au islands on Pt-Ni: an ideal trimetallic nanoframe catalyst. J Am Chem Soc, 2014, 136: 11594–11597
Hong JW, Lee SU, Lee YW, et al. Hexoctahedral Au nanocrystals with high-index facets and their optical and surface-enhanced Raman scattering properties. J Am Chem Soc, 2012, 134: 4565–4568
Chien CT, Yan JY, Chiu WC, et al. Caged Pt nanoclusters exhibiting corrodibility to exert tumor-inside activation for anticancer chemotherapeutics. Adv Mater, 2013, 25: 5067–5073
Zhang H, Jin MS, Xiong YJ, et al. Shape-controlled synthesis of Pd nanocrystals and yheir catalytic applications. Acc Chem Rev, 2013, 46: 1783–1794
Xia YN, Xiong YJ, Lim B, et al. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed, 2009, 48: 60–103
Xie SF, Choi SI, Xia XH, et al. Catalysis on faceted noble-metal nanocrystals: both shape and size matter. Curr Opin Chem Eng, 2013, 2: 142–150
Zhou KB, Li YD. Catalysis based on nanocrystals with well-defined facets. Angew Chem Int Ed, 2012, 51: 602–613
Zhu JH, Wu JJ, Liu F, et al. Controlled synthesis of FePt-Au hybrid nanoparticles triggered by reaction atmosphere and FePt seeds. Nanoscale, 2013, 5: 9141–9149
Peng ZM, Yang H. Designer platinum nanoparticles: control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today, 2009, 4: 143–164
Chen J, Lim B, Lee EP, et al. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today, 2009, 4: 81–95
Xu D, Liu ZP, Yang HZ, et al. Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum-copper nanocubes. Angew Chem Int Ed, 2009, 48: 4217–4221
Wang C, Daimon H, Onodera T, et al. A general approach to the size- and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew Chem Int Ed, 2008, 47: 3588–3591
Tsung CK, Kuhn JN, Huang WY, et al. Sub-10 nm Platinum nanocrystals with size and shape control: catalytic study for ethylene and pyrrole hydrogenation. J Am Chem Soc, 2009, 131: 5816–5822
Wang C, Daimon H, Lee YM, et al. Synthesis of monodisperse Pt nanocubes and their enhanced catalysis for oxygen reduction. J Am Chem Soc, 2007, 129: 6974–6975
Kang YJ, Murray CB. Synthesis and electrocatalytic properties of cubic Mn-Pt nanocrystals (nanocubes). J Am Chem Soc, 2010, 132: 7568–7569
Solla-Gullón J, Vidal-Iglesias FJ, Rodríguez P, et al. In situ surface characterization of preferentially oriented platinum nanoparticles by using electrochemical structure sensitive adsorption reactions. J Phys Chem B, 2004, 108: 13573–13575
Martínez-Rodríguez RA, Vidal-Iglesias FJ, Solla-Gullón J, et al. Synthesis of Pt nanoparticles in water-in-oil microemulsion: effect of HCl on their surface structure. J Am Chem Soc, 2014, 136: 1280–1283
Han SB, Song YJ, Lee JM, et al. Platinum nanocube catalysts for methanol and ethanol electrooxidation. Electrochem Commun, 2008, 10: 1044–1047
Vidal-Iglesias FJ, Solla-Gullón J, Rodríguez P, et al. Shape-dependent electrocatalysis: ammonia oxidation on platinum nanoparticles with preferential (100) surfaces. Electrochem Commun, 2004, 6: 1080–1084
Lee H, Habas SE, Kweskin S, et al. Morphological control of catalytically active platinum nanocrystals. Angew Chem Int Ed, 2006, 118: 7988–7922
Ahmadi TS, Wang ZL, Green TC. Shape-controlled synthesis of colloidal platinum nanoparticles. Science, 1996, 272: 1924–1925
Watanabe M, Motoo S. Electrocatalysis by ad-atoms: part II. Enhancement of the oxidation of methanol on platinum by ruthenium ad-atoms. J Electroanal Chem Interfacial Electrochem, 1975, 60: 267–273
Yang HZ, Zhang J, Sun K, et al. Enhancing by weakening: electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew Chem Int Ed, 2010, 49: 6848–6851
Tong YY, Kim HS, Babu PK, et al. An NMR investigation of CO tolerance in a Pt/Ru fuel cell catalyst. J Am Chem Soc, 2002, 124: 468–473
Xu D, Bliznakov S, Liu ZP, et al. Composition-dependent electrocatalytic activity of Pt-Cu nanocube catalysts for formic acid oxidation. Angew Chem Int Ed, 2010, 49: 1282–1285
Zhang J, Yang HZ, Yang KK, et al. Monodisperse Pt3Fe nanocubes: synthesis, characterization, self-assembly, and electrocatalytic activity. Adv Funct Mater, 2010, 20: 3727–3733
EI Sawy EN, Birss VI. Nano-porous iridium and iridium oxide thin films formed by high efficiency electrodeposition. J Mater Chem, 2009, 19: 8244–8252
Chen W, Chen SW. Iridium-platinum alloy nanoparticles: composition-dependent electrocatalytic activity for formic acid oxidation. J Mater Chem, 2011, 21: 9169–9178
Imbeault R, Finkelstein D, Reyter D, et al. Kinetically stable Ptx Ir100−x alloy thin films prepared by pulsed laser deposition: oxidation of NH3 and poisoning resistance. Electrochim Acta, 2014, 142: 289–298
Assumpção MHMT, da Silva SG, da Souza RFB, et al. Direct ammonia fuel cell performance using PtIr/C as anode electrocatalysts. Int J Hydrogen Energ, 2014, 39: 5148–5152
Vitse F, Cooper M, Botte GG. On the use of ammonia electrolysis for hydrogen production. J Power Sources, 2005, 142: 18–26
Endo K, Katayama Y, Miura T. Pt-Ir and Pt-Cu binary alloys as the electrocatalyst for ammonia oxidation. Electrochim Acta, 2004, 49: 1635–1638
Vidal-Iglesias FJ, Solla-Gullón J, Montiel V, et al. Screening of electrocatalysts for direct ammonia fuel cell: ammonia oxidation on PtMe (Me: Ir, Rh, Pd, Ru) and preferentially oriented Pt(100) nanoparticles. J Power Source, 2007, 171: 448–456
Freitas RG, Antunes EP, Pereira EC. CO and methanol electrooxidation on Pt/Ir/Pt multilayers electrodes. Electrochim Acta, 2009, 54: 1999–2003
Liao SJ, Holmes KA, Tsaprailis H, et al. High performance PtRulr catalysts supported on carbon nanotubes for the anodic oxidation of methanol. J Electrochem Soc, 2006, 128: 3504–3505
Shan CC, Tsai DS, Huang YS, et al. Pt-Ir-IrO2 NT thin-wall electrocatalysts derived from IrO2 nanotubes and their catalytic activities in methanol oxidation. Chem Mater, 2007, 19: 424–431
Yi QF, Chen AC, Huang W, et al. Titanium-supported nanoporous bimetallic Pt-Ir electrocatalysts for formic acid oxidation. Electrochem Commun, 2007, 9: 1513–1518
Ioroi T, Yasuda K. Platinum-Iridium alloys as oxygen reduction electrocatalysts for polymer electrolyte fuel cells. J Electrochem Soc, 2005, 152: 1917–1924
Zhong C, Hu WB, Cheng YF. Recent advances in electrocatalysts for electro-oxidation of ammonia. J Mater Chem A, 2013, 1: 3216–3238
Bunce NJ, Bejan D. Mechanism of electrochemical oxidation of ammonia, Electrochim Acta, 2011, 56: 8085–8093
Lim B, Jiang MJ, Camargo PHC, et al. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science, 2009, 324: 1302–1305
Lim B, Lu XM, Jiang MJ, et al. Facile synthesis of highly faceted multioctahedral Pt nanocrystals through controlled overgrowth. Nano Letters, 2008, 8: 4043–4047
Choi SI, Choi R, Han SW, et al. Synthesis and characterization of Pt9Co nanocubes with high activity for oxygen reduction. Chem Commun, 2010, 46: 4950–4952
Saita S, Maenosono S. Formation mechanism of FePt nanoparticles synthesized via pyrolysis of iron(III) ethoxide and platinum(II) acetylacetonate. Chem Mater, 2005, 17: 6624–6634
Chou SW, Zhu CL, Neeleshwar S, et al. Controlled growth and magnetic property of FePt nanostructure: cuboctahedron, octapod, truncated cube, and cube. Chem Mater, 2009, 21: 4955–4961
Wu JJ, Zhu JH, Zhou MG, et al. FePt concave nanocubes with enhanced methanol oxidation activity. CrysEngComm, 2012, 14: 7572–7575
Ung D, Tung LD, Caruntu G, et al. Variant shape growth of nanoparticles of metallic Fe-Pt, Fe-Pd and Fe-Pt-Pd alloys. CrystEngComm, 2009, 11: 1309–1316
Kang YJ, Pyo JB, Ye XC, et al. Synthesis, shape control, and methanol electro-oxidation properties of Pt-Zn alloy and Pt3Zn intermetallic nanocrystals. Acs Nano, 2012, 6: 5642–5647
Zhang J, Fang JY. A general strategy for preparation of Pt3d-transition metal (Co, Fe, Ni) nanocubes. J Am Chem Soc, 2009, 131: 18543–18547
Choi SI, Xie SF, Shao MH, et al. Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mg Pt for the oxygen reduction reaction. Nano Lett, 2013, 13: 3420–3425.
Zhang J, Yang HZ, Fang JY, et al. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett, 2010, 10: 638–644
Wang YX, Sun ZY, Kumbhar A, et al. Is CO adequate to facilitate the formation of Pt3M (M = Fe, Ni and Co) nanocubes? Chem Comm, 2013, 49, 3955–3957
Xiong YJ, Xia YN. Shape-controlled synthesis of metal nanostructures: the case of palladium. Adv Mater, 2007, 19: 3385–3391
Murray CB, Kagan CR, Bawendi, MG. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci, 2000, 30: 545–610
Wang ZL. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J Phys Chem B, 2000, 104: 1153–1175
Moulder JF, Stickle WF, Sobol PE, et al. Handbook of X-ray Photoelectron Spectroscopy. Minnesota: Perkin-Elmer Corporation, 1992
Marković NM, Ross PN, Surface science studies of model fuel cell electrocatalysts. Surf Sci Rep, 2002, 45: 117–229
Marković NM, Grgur BN, Ross PN. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J Phys Chem B, 1997, 101: 5405–5413
Gloaguen F, Leger J, Lamy C, et al. Platinum electrodeposition on graphite: electrochemical study and STM imaging. Electrochim Acta, 1998, 44: 1805–1816
Antolini E, Giorgi L, Pozio A, et al. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J Power Sources, 1999, 77: 136–142
Wasmus S, Vasini EJ, Krausa M, et al. DEMS-cyclic voltammetry investigation of the electrochemistry of nitrogen compounds in 0.5 M potassium hydroxide. Electrochim Acta, 1994, 39: 23–31
Gootzen JFE, Wonders AH, Visscher W, et al. A DEMS and cyclic voltammetry study of NH3 oxidation on platinized platinum. Electrochim Acta, 1998, 43: 1851–1861
Deng XH, Wu YT, He MF, et al. Electrochemical deposition of Pt particles on indium tin oxide electrode and their electrocatalytic applications in ammonia oxidation. Acta Chim Sin, 2011, 69: 1041–1046
Zhong C, Hu WB, Cheng YF. On the essential role of current density in electrocatalytic activity of the electrodeposited platinum for oxidation of ammonia. J Power Sources, 2011, 196: 8064–8072
Liu J, Zhong C, Yang Y, et al. Electrochemical preparation and characterization of Pt particles on ITO substrate: morphological effect on ammonia oxidation. Int J Hydrogen Energy, 2012, 37: 8981–8987
Liu J, Hu WB, Zhong C, et al. Surfactant-free electrochemical synthesis of hierarchical platinum particle electrocatalysts for oxidation of ammonia. J Power Sources, 2013, 223: 165–174
Du XT, Yang Y, Liu J, et al. Surfactant-free and template-free electrochemical approach to prepare well-dispersed Pt nanosheets and their high electrocatalytic activities for ammonia oxidation. Electrochim Acta, 2013, 111: 562–566
Gerischer H, Mauerer A. Untersuchungen zur anodischen oxidation von ammoniak an platin-elektroden. J Electroanal Chem, 1970, 25: 421–433
Rosca V, Koper MTM. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces. Phys Chem Chem Phys, 2006, 8: 2513–2524
Vot SL, Roué L, Bélanger D. Study of the electrochemical oxidation of ammonia on platinum in alkaline solution: effect of electrodeposition potential on the activity of platinum. J Electroanal Chem, 2013, 691: 18–27
Rosca V, Koper MTM. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim Acta, 2008, 53: 5199–5205
Vidal-Iglesias FJ, Solla-Gullón J, Pérez JM, et al. Evidence by SERS of azide anion participation in ammonia electrooxidation in alkaline medium on nanostructured Pt electrodes. Electrochem Commun, 2006, 8: 102–106
Novell-Leruth G, Valcárcel A, Clotet A, et al. DFT characterization of adsorbed NHx species on Pt(100) and Pt(111) surfaces. J Phys Chem B, 2005, 109: 18061–18069
Author information
Authors and Affiliations
Corresponding author
Additional information
Cheng Zhong became an associate professor at the State Key Laboratory of Metal Matrix Composites, Department of Materials Science & Engineering, Shanghai Jiao Tong University in 2012, and joined the Department of Materials Science & Engineering, Tianjin University in 2014. He graduated from Fudan University with a BSc in 2004 and a PhD in 2009. His recent research interests focus on developing micro/nanostructured materials for electrochemical and electrocatalysis applications.
Wenbin Hu is a professor at the Department of Materials Science & Engineering, Tianjin University. He graduated from Central-South University with a BSc in 1988. He received his MSc from Tianjin University in 1991, and PhD from Central-South University in 1994. His research interests focus on design, synthesis and characterization of advanced nanomaterials for energy storage and conversion applications.
Rights and permissions
About this article
Cite this article
Zhong, C., Liu, J., Ni, Z. et al. Shape-controlled synthesis of Pt-Ir nanocubes with preferential (100) orientation and their unusual enhanced electrocatalytic activities. Sci. China Mater. 57, 13–25 (2014). https://doi.org/10.1007/s40843-014-0010-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-014-0010-5