Abstract
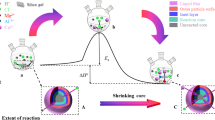
The single variable method was employed to investigate the general behavior and describe the kinetics of titanium leaching from mechanically activated titanium-bearing blast furnace slag. The optimal leaching characteristics were determined by varying the leaching temperature, leaching time, stirring intensity, hydrochloric acid solution mass fraction, and liquid-to-solid ratio (volume:mass). The optimal mechanical activation characteristics were determined using the optimal leaching process by varying the mechanical activation time, activation rotation rate, and milling ball-to-material mass ratio. The results indicated that the optimal leaching process characteristics comprise leaching temperature of 100 °C, leaching time of 120 min, stirring intensity of 600 r/min, hydrochloric acid mass fraction of 50%, and liquid-to-solid ratio of 100:1. According to the variation of titanium leaching rate from mechanically activated titanium-bearing blast furnace slag with different mechanically activation conditions, the optimal mechanical activation time, activation rotation rate, and milling ball-to-material mass ratio were determined to be 170 min, 400 r/min, and 20:1, respectively. The kinetics of titanium leaching from mechanically activated titanium-bearing blast furnace slag were effectively described by the linear relationship \(1 - \left( {1 - \alpha } \right)^{\frac{1}{3}} = kt\) and the apparent activation energy of the reaction calculated from the slope of the line is 55.52 kJ/mol, which is consistent with the characteristics of the contraction model interface chemical control step. Therefore, the shrinking core model was identified as the leaching reaction kinetics model. The XRD patterns of leaching residues of unactivated and mechanically activated slag provided evidence that mechanical activation indeed promoted the reactivity of titanium-bearing blast furnace slag and significantly improved the titanium leaching rate.
Graphical Abstract









Similar content being viewed by others
References
Lv XW, Lun ZG, Yin JQ, Bai CG (2013) Carbothermic reduction of vanadium titanomagnetite by microwave irradiation and smelting behavior. ISIJ Int 53(7):1115–1119
Chen DS, Zhao LS, Liu YH, Qi T, Wang JC, Wang LN (2013) A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes. J Hazard Mater 244–245:588–595
Fu WG, Wen YC, Xie HE (2011) Development of intensified technologies of vanadium-bearing titanomagnetite smelting. J Iron Steel Res 18(4):7–10
Zhao LS, Wang LN, Qi T, Chen DS, Zhao HX, Liu YH (2014) A novel method to extract iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 149:106–109
Zheng FQ, Chen F, Guo YF, Jiang T, Travyanov AY, Qiu GZ (2016) Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag. J Miner Met Mater Soc 68(5):1–9
Zhang YM, Wang LN, Chen DS, Wang WJ, Liu YH, Zhao HX, Qi T (2018) A method for recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite. Int J Miner Metall Mater 25(2):131–144
Nie WL, Wen SM, Feng QC, Liu D, Zhou YW (2020) Mechanism and kinetics study of sulfuric acid leaching of titanium from titanium-bearing electric furnace slag. J Market Res 9(2):1750–1758
Bian ZZ, Feng YL, Li HR, Wu H (2020) Efficient separation of vanadium, titanium and iron from vanadium-bearing titanomagnetite by pressurized pyrolysis of ammonium chloride-acid leaching-solvent extraction process. Sep Purif Technol 255:117169
Zhang YM, Yi LY, Wang LN, Chen DS, Wang WJ, Liu YH, Zhao HX, Qi T (2017) A novel process for the recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite: sodium modification-direct reduction coupled process. Int J Miner Metall Mater 5:8
Sun Y, Zheng HY, Dong JH, Shen HM (2011) Fundamental study on extracting vanadium from vanadium-bearing titanomagnetite. Adv Mater Res 284–286:1170–1173
Godínez AC (2003) Comparative study of solvent extraction of vanadium from sulphate solutions by primene 81R and alamine 336. Miner Eng 16(3):291–294
Moskalyk RR, Alfantazi AM (2003) Processing of vanadium: a review. Miner Eng 16(9):793–805
Sadykhov GB, Karyazin IA (2007) Titanium-vanadium slags produced upon the direct reduction of iron from titanomagnetite concentrates. Russ Metall 6:447–454
Yang DM, Peng MF, Zhu SY, Hua YX (2006) Preparing VO2 powder using leach solution of vanadium slag. Iron Steel Vanadium Titanium 27:59–63 (In Chinese with English abstract)
Xiong Y, Li C, Liang B, Xie J (2008) Leaching behavior of air cooled Ti-bearing blast-furnace slag in hydrochloric acid. Chin J Nonferr Met 18(3):557–563
Sasikumar C, Rao DS, Srikanth S, Ravikumar B, Mukhopadhyay NK, Mehrotra SP (2004) Effect of mechanical activation on the kinetics of sulfuric acid leaching of beach sand ilmenite from Orissa, India. Hydrometallurgy 2004(75):189–204
Wang M, Tan J, Chen QY, Hu HP, Yao TM (2012) Effect of oxidization-reduction-mechanical activation process on leaching behavior of panxi ilmenite. Nonferr Met 5:1–4
Xing XD, Zhang JL, Wang ZY, Liang JQ, Liu YR (2015) Enrichment research on direct reduction melting and separation TiO2 of vanadium titano-magnetite slag by acid leaching. Light Met 11:45–49
Li C, Liang B, Liang XM (2005) Leaching kinetics of mechanically activated ilmenite ore. J Sichuan Univ 37(1):35–38
Zhang K, Cheng Y, Li W et al (2019) Microcrystalline characterization and morphological structure of tectonic anthracite using XRD, liquid nitrogen adsorption, mercury porosimetry, and micro-CT. Energy Fuels 33:10844–10851
Chen Q, Yin Z, Zhang P et al (2002) The oxidation behavior of unactivated and mechanically activated sphalerite. Metall Mater Trans B 33(6):897–900
Wang TH, Navarrete-LóPez AM, Li S, Dixon DA, Gole JL (2010) Hydrolysis of TiCl4: initial steps in the production of TiO2. J Phys Chem A 114:7561
Gu SX, Luo XH (1987) Dissolution of metatitanic acid in hydrochloric acid. Chin J Rare Met 96(2):96–100 (In Chinese)
Wu EH, Li J, Hou J, Xu Z, Liu QS, Huang P, Chen HZ (2019) Experimental study on preparation of ilmenite concentrate by hydrochloric acid leaching of titanium middling ore at atmospheric pressure. Multipurp Util Miner Resour 03:48–51 (In Chinese with English abstract)
Zhu* S, Hu J, Zhang C, Li S, An N (2022) Study on optimization and mechanism of mechanical activation process of titanium-bearing blast furnace slag. J Mater Res Technol 19: 3130–3144
Xu S, Huang Z (1993) The kinetics of Panzhihua ilmenite leaching with sulfuric acid. Min Metall Eng 13(1):44–48
Sohn HY, Wadsworth ME (1979) Rate processes of extractive metallurgy. Plenum Press, New York, pp 135–143
Habashi F (1969) Principles of extractive metallurgy, general principles, 2nd edn. Gordon and Breach Science Publishers Inc, New York, pp 111–169
Acknowledgements
This work was financially supported by Guizhou Provincial Science and Technology Projects (Guizhou Science and Technology Foundation [2019]1292), Liupanshui Metallurgical Solid Waste Recycling and Environmental Protection Technology Innovation Team (52020-2019-05-08), High-level talent research start-up fund of Liupanshui Normal University (LPSSYKYJJ201809), Key field project of Natural Science Research of Guizhou Provincial Department of Education (Guizhou Education KY[2020]049), Guizhou Liupanshui Normal University academician workstation (Guizhou Science and Technology Platform Talent [2019]5604), Guizhou Key Laboratory of clean coal utilization (Guizhou Science and Technology Platform Talent [2020]2001), Development project of young scientific and technological talents of Guizhou Provincial Department of Education (Qian Jiao he KY Zi[2017]257), and Liupanshui science and Technology Bureau project (52020-2018-04-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Hu, J., Zhang, C. et al. Process Optimization and Kinetics of Titanium Leaching from Mechanically Activated Titanium-Bearing Blast Furnace Slag. J. Sustain. Metall. 9, 230–239 (2023). https://doi.org/10.1007/s40831-022-00640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00640-7