Abstract
We have measured NOx conversions and N2O productions over Fe-BEA and Cu-SAPO catalysts and over their sequential arrangements under Enhanced SCR conditions, resulting from the addition of an aqueous solution of ammonium nitrate (AN) to the typical Standard SCR feed stream, and we have compared them to those observed under Standard and Fast SCR conditions. The expected strong enhancement of the poor low temperature activity of the Fe-BEA catalyst was confirmed: both NH3 and NOx conversions and N2O formations similar to those of the Fast SCR reaction were achieved when cofeeding ammonium nitrate. On the other hand, the Cu-SAPO efficiency was drastically decreased by the addition of AN at low temperatures, possibly due to trapping of the ammonium nitrate salt within the SAPO zeolite, characterized by smaller pores than those of the BEA zeolite. The Cu-SAPO performances were recovered only at T > 250 °C with a huge release of N2O due to the thermal decomposition of AN. The combined system with the Fe-zeolite sample placed upstream of the Cu-zeolite also exhibited outstanding low temperature deNOx performances, with even lower N2O production than over the Fe-zeolite only at the same Enhanced SCR (E-SCR) conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The current well-established solution for the NOx emission control on Diesel and/or natural gas lean-burn engines is represented by the NH3 selective catalytic reduction (NH3 SCR) [1, 2]. The NOx abatement occurs over state-of-the-art catalysts, such as Fe-, Cu-zeolite and V-based catalysts [2,3,4,5,6,7,8,9,10,11,12,13], by means of the injection of urea, which, once decomposed to NH3, can react with NOx producing harmless N2 and H2O, according to the main SCR reactions
The deNOx performance of the SCR systems is strictly connected to the temperature of the exhaust gases. One big challenge is the improvement of the efficiency when the temperatures are too low for the SCR catalysts to be active and for the urea to effectively decompose. Moreover, the development of more efficient Diesel engines is leading to operating conditions with high contents of oxygen. Thus, such increasingly efficient engines produce higher amounts of NOx as well as exhaust gases with increasingly lower temperatures, aggravating the task of the SCR converter. A way to maximize the NOx conversion to N2 in the mobile applications is by increasing the NO2/NO molar feed ratio in order to promote the Fast SCR reaction (2). Since the NOx gases, produced during the combustion, are composed mainly by NO (95%), the Standard SCR reaction (1) is the main reaction involved in the NOx control, which is unfortunately characterized by a significantly lower efficiency than the Fast SCR (2) in the low temperature range [2]. The NO2/NO molar feed ratio can be modified in the catalytic oxidation step mainly dedicated to the abetment of unburned hydrocarbon and CO, the Diesel Oxidation Catalyst (DOC) or/and Methane Oxidation Catalyst (MOC). The presence of NO2 in the exhausts allows the improvement of the deNOx efficiency of the SCR systems in the low T range, especially when the NO2/NO molar ratio is 1/1 and the Fast SCR can take place (2). However, the oxidation activity of the DOC/MOC is strongly dependent on temperature and flow rate of the exhaust gases and, consequently, the optimal NO2/NO feed ratio cannot be guaranteed for all the possible engine operating conditions [14].
In the last years, another catalytic route was proposed to overcome the issues connected to the presence of a proper amount of NO2 in the gas mixture necessary to boost the typical poor deNOx activity of Fe-zeolite or V-based catalysts in the low T range. This involves the addition of an aqueous solution of ammonium nitrate (NH4NO3) to a NO–NH3 containing gas mixture, which determines the occurrence of the so-called Enhanced SCR reaction (3)
The E-SCR reaction was previously studied on Fe-zeolite and V-based catalysts [14,15,16,17,18] and it was demonstrated to be associated with superior NO reduction efficiencies in the 200–350 °C T range with respect to the Standard SCR reaction, allowing to approach the desired higher Fast SCR activity. It is worth emphasizing that this solution does not require the oxidation of NO to NO2 upstream of the SCR converter since this oxidation is directly entrusted to the ammonium nitrate itself according to the reaction (4)
The produced NO2 is, then, able to react further with the remaining NO and with NH3 in accordance with the Fast SCR Reaction (5): the Enhanced SCR reaction (6), therefore, is the sequential combination of these two catalytic steps: (4) + (2) [17]. The Enhanced SCR and the Fast SCR are characterized by the same reaction mechanism, which differs only in their first step, existing a direct equivalence between AN and NO2 [17].
The ammonium nitrate feed can be obtained by injecting or vaporizing an aqueous solution of ammonium nitrate upstream of the SCR converter, together with the urea solution: indeed, single aqueous solutions containing both the reducing agent (urea) and the oxidizing additive (ammonium nitrate) are commercialized and available for this purpose [14].
This work aims at brushing up this concept since it gives interesting perspectives for the enhancement of the critical low temperature deNOx activity without necessarily relying on the generation of NO2 in the DOC/MOC. Since it was demonstrated to be a concept applicable to two classes of commercial SCR catalysts (V-based systems and Fe-exchanged zeolites), here we complete the study by investigating also the deNOx performances of a Cu-promoted zeolite catalyst and of combined Fe- and Cu-zeolite systems under Enhanced SCR conditions, eventually comparing them with the Standard and Fast SCR data.
2 Experimental
Steady-state Standard SCR, Fast SCR and Enhanced SCR catalytic activity tests were performed over two NH3-SCR catalysts, namely a thermally stable Cu-SAPO and a Fe-BEA, supplied in the shape of coated monoliths by Dinex Finland [19]. The Fe-BEA catalyst was coated onto a cylindrical rolled metallic substrate made of flat and corrugated thin (50 μm) AlCr foils (cell density of 600 cpsi). The coating load was about 150 g/L (40 g/m2). The dimensions of the tested sample were L = 20 mm, D = 12.5 mm, V = 2453 mm3. The Cu-SAPO catalyst was tested in the form of a coated ceramic honeycomb (400 cpsi). The coating load was about 140 g/L. The dimensions of the tested sample were L = 41.8 mm, h = 7.7 mm, w = 7.7 mm, V = 2478 mm3. The combined systems were realized putting both catalysts in series, so that 50% of the total catalyst volume was composed by the Cu-sample and 50% by the Fe-sample. The molar Si/Al2 ratio in the zeolites was in the range of 25–40 to maintain a good hydrothermal stability. A small amount (< 15wt% of final coating) of binder was added in the coating process. The catalysts were hydrothermally (HT) aged at 700 °C for 20 h in air flow with 10% of water [2, 19]. Data on the sulphur resistance of both catalysts are also given in [19].
The experimental equipment used to carry out this work was described in our previous work [2]. Steady-state runs were performed in order to investigate the catalytic activities in a wide T range (150–550 °C), using defined reactant feed concentrations and temperature steps. The feed composition was representative of real after treatment systems: NH3 = 500 ppm, NOx = 500 ppm (NO2/NOx = 0–0.5), O2 = 5% (v/v), H2O = 5% (v/v) and balance N2. Under Enhanced SCR conditions an aqueous solution of NH4NO3 was fed to the reactor: the ammonium nitrate feed concentration level of 200 ppm was achieved by properly diluting a master 2.5 M solution. Either liquid water or the AN + H2O solution was metered by a volumetric piston pump (Gilson model 305): the feed rate was around 0.025 ml/min +/− 0.0001 for GHSV = 75,000 h−1. Afterwards, the liquid feed was vaporized in a hot pipeline kept at 190 °C, and then mixed with the other gaseous species and fed to the reactor. All the gaseous species (except N2 and AN) were continuously monitored at the reactor outlet by a FT-IR gas analyser (Bruker MATRIX MG5).
3 Results
3.1 Enhanced SCR on Fe-BEA
In our previous work [2], we reported the investigation of two commercial SCR catalysts, a Fe-BEA and Cu-SAPO, under typical exhaust aftertreatment conditions for natural gas or duel fuel vehicles. Moreover, we demonstrated that the sequential arrangement of the two Fe- and Cu- monolith catalysts allowed to overcome the issues connected to the use of only one of these metal-promoted zeolite catalysts under Standard SCR conditions, namely the poor low-T deNOx activity typical of Fe-zeolite catalysts and the lower selectivity in the high-T range of Cu-zeolites. Their combination (Fe followed by Cu), indeed, permitted to mitigate these drawbacks keeping only the advantages of the two catalytic systems, i.e. the outstanding low-T deNOx efficiency of the Cu-zeolite and the high-T selectivity and activity of the Fe-zeolite. The Cu-zeolite, in fact, placed after the Fe-zeolite, compensated the poor Fe- catalyst activity at low temperature, thus achieving greater overall deNOx performances under Standard SCR conditions.
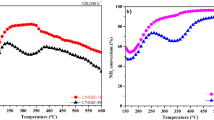
An alternative way to improve the low-T deNOx efficiency of Fe-zeolite systems, however, was already proposed and discussed in the literature [14,15,16,17,18]: the addition of AN to the feed stream, indeed, was demonstrated to increase significantly the low-T Standard SCR NOx conversions over Fe-zeolite catalysts. Figure 1 compares the NOx steady-state conversions collected under Standard SCR conditions (GHSV = 75,000 h−1, NH3 = 500 ppm, NO = 500 ppm, H2O = 5% v/v, O2 = 5% v/v) over the Fe-BEA and Cu-SAPO samples and their sequential combination (Fe + Cu) (already shown in [2]) with the NOx conversions achieved on the Fe-BEA sample under the same Standard SCR conditions when 200 ppm of NH4NO3 were added to the gas feed mixture (Enhanced SCR). Figure 1 confirms that the AN dramatically boosts the NOx conversion over the Fe-zeolite at temperatures below 300 °C, matching the well-known outstanding Standard SCR activity of the Cu-zeolite catalyst, or even overcoming it in the intermediate temperature range. Moreover, the Enhanced SCR activity of the Fe-zeolite sample outperformed the Standard SCR activity of the combined Fe- + Cu-zeolite system (Fig. 1).
Comparison of NOx conversion under Std SCR conditions on Fe-BEA, Cu-SAPO, and Fe-+Cu-zeolite catalysts and of NOx conversion under E-SCR conditions on Fe-zeolite catalyst. GHSV = 75,000 h−1, NH3 = 500 ppm NO = 500 ppm, O2 = 5% v/v, H2O = 5% v/v, Trange = 150–550 °C. Std SCR = NH4NO3/NO = 0; E-SCR = NH4NO3/NO = 2/5
Figure 2 directly demonstrates the ammonium nitrate addition effect on the Fe-zeolite sample by comparing the steady-state NOx conversions and N2O productions measured under Fast SCR, Standard SCR and Enhanced SCR conditions. As previously noted, the Enhanced SCR reaction resulted in a significantly higher deNOx activity at low temperatures with respect to the Standard SCR reaction, approaching the activity of Fast SCR (Fig. 2a). In these tests, the adopted AN feed concentration was 200 ppm. As demonstrated by Marchitti et al. [17], the ammonium nitrate is able to oxidize NO to NO2, according to reaction (4). Then the co-presence of NO, NO2 and NH3 allows the occurrence of the Fast SCR reaction. Considering that all the ammonium nitrate injected in our system oxidized NO to NO2, the resulting NO2/NOx ratio was 2/5, a little lower than the ratio used under Fast SCR conditions (1/2), consistent with our E-SCR experiments remaining below the Fast SCR activity.
Standard SCR, Fast SCR and Enhanced SCR reactions on Fe-BEA: a NOx steady-state conversions, b N2O steady-state productions, c N2 Selectivity. GHSV = 75,000 h−1, NH3 = 500 ppm NOx = 500 ppm, O2 = 5% v/v, H2O = 5% v/v, Trange = 200–550 °C. Std SCR: NO2/NOx = 0, NH4NO3/NOx = 0; Fast SCR: NO2/NOx = 0.5, NH4NO3/NOx = 0; E-SCR: NO2/NOx = 0, NH4NO3/NO = 2/5
Even if the AN dosing is able to improve the NOx conversions, the N2O formation is a delicate aspect that needs to be controlled, being a strongly undesired by-product due to its high greenhouse potential. In fact, ammonium nitrate could be involved in other side reactions which are not advantageous for the deNOx performances but, on the contrary, can contribute to the emission of more pollutants, losing that benefit obtained by injecting nitrate species for the reduction of NOx. The ammonium nitrate, in fact, can decompose according to the following reactions [18]:
Reactions (7) and (8) determine the production of NH3 and NO2. In our systems, we observed a negligible formation of NO2, which suggests that the nitrate ad-species possibly formed by reaction (7) directly participate in SCR reactions [18]. On the other hand, traces of N2O were detected, which are shown in Fig. 2b and compared with the amount of N2O emitted under Standard SCR, Fast SCR conditions in the case of Fe-BEA. Figure 2c shows the N2 selectivities computed as [1–2*N2Oproduction/(NH3 + NO+NH4NO3)consumption], i.e. assuming that N2O is the only side product of the SCR process. Even though the Standard SCR reaction is characterized by the worst activity performance, the NO-NH3 reacting system shows the highest N2 selectivity at any investigated temperature. The Fast and the E-SCR reactions, instead, contribute to a significant N2O production (Fig. 2b). Moreover, they show also the same selectivity up to 450 °C, while at higher temperature the E-SCR reaction recovers the Standard SCR selectivity (Fig. 2c).
3.2 Enhanced SCR on Cu-SAPO
The Enhanced SCR reaction was carried out for the first time on a Cu-zeolite catalyst, specifically a Cu-SAPO sample, and compared with the data collected under Standard and Fast SCR conditions. The steady-state NOx conversions and N2O productions observed for the three reactions are compared in Fig. 3. For the Cu-SAPO catalyst, the situation was reversed with respect to Fe-BEA: at the lowest investigated temperature, the AN dosing worsened the good deNOx efficiency achieved both under Standard and Fast SCR conditions. The negative impact of the addition of the AN aqueous solution could be ascribed to the porous structure of the zeolite itself. It is well known that the SAPO chabazite framework is associated with pores with a smaller size compared, for example, to those of the BEA zeolite [20]. At low temperatures (≤ about 200 °C), the ammonium nitrate salt can be trapped within the small pores of the zeolite, covering the active sites and thus inhibiting the SCR activity. Indeed, on increasing the temperature the activity was recovered, consistent with the fact that the ammonium nitrate was thermally decomposed according to reaction (9) releasing large amounts of N2O, as shown in Fig. 3b.
Standard SCR, Fast SCR and Enhanced SCR reactions on Cu-SAPO catalysts: a NOx steady-state conversions, b N2O productions, c N2 selectivity. GHSV = 75,000 h−1, NH3 = 500 ppm NOx = 500 ppm, O2 = 5% v/v, H2O = 5% v/v, Trange = 200–550 °C. Std SCR: NO2/NOx = 0, NH4NO3/NOx = 0; Fast SCR: NO2/NOx = 0.5, NH4NO3/NOx = 0; E-SCR: NO2/NOx = 0, NH4NO3/NO = 2/5
Accordingly, the N2 selectivity under E-SCR conditions remained lower than under Standard and Fast SCR conditions (Fig. 3c), confirming a negative impact of the NH4NO3 addition on the deNOx performance of the Cu-SAPO catalyst.
3.3 Enhanced SCR Fe-BEA + Cu-SAPO: Effect of the Sequential Arrangement
We verified and shown in Fig. 1 that the addition of a Cu-SAPO catalyst after the Fe-BEA monolith can improve the NOx removal efficiency over that of the Fe-zeolite alone. However, since in the low-T range, the combined system exhibited lower NOx conversions under Std SCR conditions with respect to those achieved on the only Fe-zeolite catalyst under Enhanced SCR conditions; the very good activity of the combined system could be, theoretically, further improved by dosing ammonium nitrate and thus running the Enhanced SCR reaction over it. For the Fe-+Cu-zeolite sequential system, Fig. 4a compares the Standard SCR NOx conversions, already seen in Fig. 1, with those collected upon addition of 200 ppm of NH4NO3 to the Std SCR conditions (E-SCR) and those obtained under the Fast SCR conditions. This plot proves that the combination of these two strategies, namely the AN boosting effect and the combination of Fe and Cu catalysts, can enable outstanding deNOx performance, already at 200 °C, even better than those obtained on the only Fe catalyst (at 200 °C the NOx conversions is about 80% against the 86% of Fe-+Cu). In addition, this combined catalytic system is beneficial also in terms of N2O production: Fig. 4b illustrates, as seen for the Fe-zeolite catalyst, that E-SCR and Fast SCR reactions are characterized by similar N2O formations and, consequently, similar N2 selectivities (Fig. 4c). However, the measured N2O concentration was always lower with respect to those detected on the only Fe-zeolite samples, confirming, also for this aspect, that the sequential arrangement is the best choice towards this application.
Standard SCR, Fast SCR and Enhanced SCR reactions on the combined system Fe- + Cu-zeolite catalysts: a NOx steady-state conversions, b N2O steady-state productions, c N2 Selectivity. GHSV = 75,000 h−1, NH3 = 500 ppm NOx = 500 ppm, O2 = 5% v/v, H2O = 5% v/v, Trange = 200–550 °C. Std SCR: NO2/NOx = 0, NH4NO3/NOx = 0; Fast SCR: NO2/NOx = 0.5, NH4NO3/NOx = 0; E-SCR: NO2/NOx = 0, NH4NO3/NO = 2/5
In our previous work [2], we checked also that the correct positioning of the Fe and Cu samples in the sequential arrangement is crucial in order to generate a synergy between the two catalysts. Under Standard SCR conditions, we observed a loss of selectivity when the Cu catalyst was placed prior to the Fe-sample, while in the low-T region, we observed similar behaviours. Consequently, our investigation was focused on the Fe- + Cu-zeolite combined arrangement. In this work, we have studied the effect of the catalyst order on the Enhanced SCR performances, too. Figure 5 illustrates the sequential arrangement effect on the deNOx performances under the Enhanced SCR conditions. Clearly, when the Cu-zeolite was placed upstream the beneficial effect of the ammonium nitrate addition was lost, the NOx conversions (Fig. 5a) being even worse than those observed under Standard SCR conditions (no AN) on the Cu catalyst alone (Fig. 1), on the Fe-+ Cu samples (Fig. 1) and on Cu-+Fe-samples (Fig. 1 in [2]). Moreover, even if in the low-T range the N2O production on the Cu + Fe-sample is lower than that on the dual sample with reverse order (Fig. 5b), the two sequential combinations are similar in terms of N2 selectivity (99% vs. 98%) (Fig. 5c). In the high-T region, instead, the Fe-+Cu- sample overcomes the other in terms of N2 selectivity and minimum N2O formation.
Sequential arrangement effect (Fe-+Cu-zeolite vs. Cu-+Fe-zeolite) on the deNOx performance under Enhanced SCR conditions: a NOx steady-state conversions, b N2O steady-state productions, c N2 selectivity. GHSV = 75,000 h−1, NH3 = 500 ppm NO = 500 ppm, O2 = 5% v/v, H2O = 5% v/v, NH4NO3 = 200 ppm, Trange = 200–550 °C
Such a negative impact of the AN dosing observed on Cu-SAPO in the previous section is in turn reflected on the sequential configuration in which the Cu-zeolite was placed before the Fe-zeolite (Fig. 5). Under Standard SCR conditions, the two configurations worked in a similar way in the low-T range [2]: in both cases, in fact, most of the reactants were converted over the Cu-zeolite catalyst. Indeed, when the Fe-BEA sample was placed upstream, being its activity lower than the following Cu-SAPO, the gases reacted in the second monolith sample. In the case of the reverse configuration, instead, NO and NH3 were consumed in the upstream Cu-zeolite section while the following Fe-zeolite was hardly utilized. In the E-SCR reaction, however, the order of the two samples plays instead an important role. Indeed, being the Fe-BEA activity significantly promoted by the AN addition, when placed upstream the Fe-zeolite was able to convert most of the reactants, obtaining the good performances shown in Fig. 1. When instead Cu-SAPO, which is adversely affected by AN, as observed in Fig. 3, was placed upstream, ammonium nitrate blocked the active sites at low temperature and in turn the consumption of the reactants moved to the second section, i.e. the Fe-BEA catalyst, which at those temperatures and without all the available ammonium nitrate feed showed its poor deNOx efficiency.
Accordingly, the E-SCR reaction represents a promising and easy method to improve the activity of metal-promoted zeolite catalysts which are characterized by a poor activity in the low-T range, as seen for the Fe-BEA sample. However, it is important to pay attention to the zeolite structure: the smaller the pore size of the zeolite framework, the worse the performances.
4 Conclusions
In this work, the cofeed of ammonium nitrate according to the Enhanced SCR reaction was demonstrated to be an effective method to improve the typically poor low-T activity of NH3-SCR Fe-zeolite catalysts. The dosage of an aqueous solution containing ammonium nitrate to Standard SCR conditions allowed to boost the overall NOx conversion via the NO oxidation to NO2 enabled by the ammonium nitrate, reaction (4). Once NO2 is formed, the Fast SCR reaction can take place thanks to the co-presence of NO, NO2 and NH3. On the other hand, the Enhanced SCR conditions had a negative impact on the Cu-SAPO catalyst: the addition of ammonium nitrate to a catalyst based on a small-pore zeolite determined a dramatic loss of activity in the low temperature region and a huge release of N2O due to the unselective thermal decomposition of NH4NO3 salt deposited within the zeolite structure. On the other hand, the sequential arrangement of a Fe-BEA catalyst followed by the Cu-SAPO sample under the E-SCR conditions showed outstanding performances, close to those corresponding to Fast SCR conditions even in the absence of NO2 in the feed stream.
References
Krocker, O.: Selective catalytic reduction of NOx. Catalysts. 8(10), 459 (2018)
Villamaina, R., Nova, I., Tronconi, E., Maunula, T., Keenan, M.: The effect of CH4 on NH3-SCR over metal-promoted zeolite catalysts for lean-burn natural gas vehicles. Top. Catal. 61(18-19), 1974–1982 (2018)
Boron, P., Rutkowska, M., Gil, B., Marszalek, B., Chmielarz, L., Dzwigaj, S.: Experimental evidence of the mechanism of selective catalytic reduction of NO with NH3 over Fe-containing BEA zeolites. Chem. Sus. Chem. 12(3), 692–705 (2019)
Xin, Y., Li, Q., Zhang, Z.: Zeolitic materials for DeNOx selective catalytic reduction. Chem. Cat. Chem. 10, 29–41 (2018)
Gao, F., Wang, Y., Washton, N.M., Kollar, M., Szanyi, J., Peden, C.H.F.: Effects of alkali and alkaline earth cocations on the activity and hydrothermal stability of Cu/SSZ-13 NH3-SCR catalysts. ACS Catal. 5(11), 6780–6791 (2015)
Wang, L., Li, W., Qi, G., Weng, D.: Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 289, 21–29 (2012)
Colombo, M., Koltsakis, G., Nova, I., Tronconi, E.: Modelling the ammonia adsorption-desorption process over an Fe-zeolite catalyst for SCR automotive applications. Catal. Today. 188(1), 42–52 (2012)
Shakya, B.M., Harold, M.P., Balakotaiah, V.: Simulations and optimization of combined Fe- and Cu-zeolite SCR monolith catalyst. Chem. Eng. J. 278, 374–384 (2015)
Fickel, D.W., D’Addio, E., Lauterbach, J.A., Lobo, R.F.: The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B. 102(3-4), 441–448 (2011)
Kamasamudram, K., Currier, N.W., Chen, X., Yezerets, A.: Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol. Catal. Today. 151(3-4), 212–222 (2010)
Metkar, P.S., Harold, M.P., Balakotaiah, V.: Experimental and kinetic modelling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu- zeolite monolithic catalysts. Chem. Eng. Sci. 87, 51–65 (2013)
Metkar, P.S., Salazar, N., Muncrief, R., Balakotaiah, V., Harold, M.P.: Selective catalytic reduction of NO with NH3 on iron zeolite monolithic catalysts: steady state and transient kinetics. Appl. Catal. B. 104(1-2), 110–126 (2011)
Wang, D., Zhang, L., Li, J., Kamasamudram, K., Epling, W.S.: NH3-SCR over Cu/SAPO-34 – Zeolite acidity and Cu structure changes as function of cu loading. Catal. Today. 231, 64–74 (2014)
Forzatti, P., Nova, I., Tronconi, E.: Enhanced NH3 selective catalytic reduction for NOx abatement. Angew. Chem. Int. Ed. 48(44), 8366–8369 (2009)
Forzatti, P., Nova, I., Tronconi, E., Kustov, A., Thøgersen, J.R.: Effect of operating variables on the enhanced SCR reaction over a commercial V2O5 -WO3/TiO2 catalyst for stationary applications. Catal. Today. 184(1), 153–159 (2012)
Forzatti, P., Nova, I., Tronconi, E.: Removal of NOx from diesel exhausts: the new “enhanced NH3-SCR” reaction. SAE Technical Paper 2010-01-1181 (2010)
Marchitti, F., Barker Hemings, E., Nova, I., Forzatti, P., Tronconi, E.: Enhancing the low-T NH3-SCR activity of a commercial Fe-zeolite catalyst by NH4NO3 dosing: an experimental and modeling study. Emiss. Control Sci. Technol. 2(1), 1–9 (2016)
Forzatti, P., Nova, I., Tronconi, E.: New “enhanced NH3-SCR” reaction for NOx emission control. Ind. Eng. Chem. Res. 49(21), 10386–10391 (2010)
Maunula, T., Wolff, T.: Durable Copper and Iron SCR Catalysts for Mobile Diesel and Dual-Fuel Applications. SAE Technical Paper 2016-01-2214 (2016)
Ruggeri, M.P., Nova, I., Tronconi, E., Collier, J., York, A.P.E.: Structure-activity relationship of different Cu-zeolite catalysts for NH3-SCR. Top. Catal. 59(10-12), 875–881 (2016)
Funding
The research leading to these results has received funding from the European Community’s Horizon 2020 Programme under grant agreement No. 653391 (HDGAS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Villamaina, R., Nova, I., Tronconi, E. et al. Effect of the NH4NO3 Addition on the Low-T NH3-SCR Performances of Individual and Combined Fe- and Cu-Zeolite Catalysts. Emiss. Control Sci. Technol. 5, 290–296 (2019). https://doi.org/10.1007/s40825-019-00140-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-019-00140-3