Abstract
A liquid-quench-based experimental method for the determination of even minimal vanadium and tungsten emissions from selective catalytic reduction (SCR) catalysts by an inductively coupled plasma optical emission spectrometer (ICP-OES) was developed. The method was applied to a commercial EuroV SCR catalyst (V2O5/WO3-TiO2) with usual operation temperatures below 450 °C, which was on purpose operated at out-of-spec temperatures above 550 °C in the laboratory. It was observed that the emissions of vanadium exponentially increase with temperature but the absolute concentrations if calculated to gas phase are very small and still below legal limits of health organizations such as NIOSH. Further, it could be shown that with heating of the same catalyst sample up to 100 h, the emissions increase before reaching a stable level that could be up to four times higher than the emission level in the first 24 h. However, the catalyst was already heavily thermally damaged due to a loss of BET surface of over 50 % compared to the fresh catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vanadia-based catalysts have been applied since decades as active, stable, and cost-efficient standard catalysts for the selective catalytic reduction (SCR) of NO x in lean exhaust gases in power plants. In the recent years, they have also been applied for NO x reduction in heavy-duty vehicles. With the introduction of tight emission limits for non-road machineries and stationary engines, vanadia-based catalysts will also be applied in SCR systems for these applications. Although alternative catalyst systems have been developed, the long-term experience and the resistance to sulfur poisoning are decisive arguments for vanadia-based catalysts in many applications. There is still some concern with respect to the emission of vanadium from the catalyst during operation at elevated temperatures as some vanadium compounds, especially vanadium pentoxide (V2O5), are considered to be potentially toxic or carcinogenic [1]. Emissions from exhaust gas catalysts play a minor role or are even negligible for the background concentration of vanadium compounds in general. In rural areas where the natural sources such as continental dust (weathering), marine aerosols, and, to a low extent, volcanic emission dominate, background concentrations between 0.3 and 5 ng/m3 air have been measured [2]. In cities where the vanadium emissions are dominated by combustion of coal and heavy fuel oil in industrial burners and by metallurgical processes [3–5], concentrations from around 11 ng/m3 air up to around 600 ng/m3 air in heavily industrialized areas can be found in the USA [1].
Hu et al. [6] investigated the vanadium emissions from a heavy-duty vehicle equipped with a combined DPF/SCR system (diesel particulate filter and vanadia-based selective reduction catalyst) on a dynamometer. Based on particulate investigation, they found emissions of 560 ± 265 ng/km for a cruise and 210 ± 120 ng/km for an urban driving cycle. By comparison with another study [7], they concluded that the tailpipe particulates contain two to three times less vanadium than ambient particulate matter collected during roadway tunnel studies.
The same group investigated in a follow-up study the chemical speciation of vanadium in particulate matter from heavy-duty vehicles [8]. They found total vanadium emissions from a vehicle equipped with a vanadium-based retrofit SCR system in a dynamometer test of 206 ± 17 ng/mile. Surprisingly, when testing a vanadium-free zeolite-based system, vanadium emission in the range of one fourth is already observed (55 ± 12 ng/mile). During a cruise, a different driving cycle, the V emissions of the zeolite system reach even half of the V-SCR, namely 99 ± 12 ng/mile. The problem of baseline is additionally illustrated by running a different engine of the same piston displacement at a cruise without any after-treatment system: the blank value reaches 111 ± 27 ng/mile. The authors contribute the high values even in the case of a vanadium-free exhaust system to vanadium in lubrication oil.
Chapman [9] developed a methodology to determine vanadium and tungsten emissions from vanadia-based catalysts by adsorption on an alumina particle bed downstream of the catalyst and subsequent dissolution of the adsorber. The vanadium/tungsten content of the resulting solution was determined by an inductively coupled plasma optical emission spectrometer (ICP-OES). He found that neither V nor W was found downstream of the catalyst in the absence of water. With a model exhaust gas containing 5 vol% water, he found during tests at 750 °C (far above the operation temperature of SCR catalysts) that V emissions are far below equilibrium while tungsten emissions reach equilibrium values according to the exponential increase of vapor pressure of hydroxide formation.
The study presented here aimed at determining V and W emissions from vanadia-based catalysts to be expected when the catalyst accidentally reaches temperatures above the standard operation conditions (usually engines are limited to 550 °C peak temperature by engine control). A dedicated laboratory test procedure to find even small emissions was developed. For this purpose, the exhaust gas emissions from a relatively big catalyst sample were collected by liquid absorption. This allowed the accumulation during 24 h by liquid recirculation and therefore for lower limits of detection and quantification by ICP-OES.
2 Experimental
2.1 Experimental Setup and Catalyst Testing Procedure
The catalyst investigated in this study was a commercial EuroV SCR catalyst (V2O5/WO3-TiO2) with normal operation temperatures below 450 °C. For developing the testing procedure, a catalyst was chosen which was designed for operation with peak temperatures below 550 °C and from which V emissions could be expected at high temperatures. As reported in literature [9], other preparation technologies for vanadium catalysts can produce more stable systems where much lower emissions can be expected.
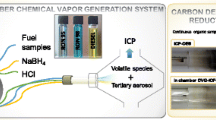
A cylindrical piece of the catalyst (length 56 mm, diameter = 21 mm, volume = 19.4 cm3) was contacted in an isothermal reactor with 582 L/h at STP of a model gas, resulting in a GHSV of 30,000/h. The setup is shown in Fig. 1. The gas consisted of N2 and 10 % O2, 5 % H2O, 500 ppm NO, 500 ppm NH3, 200 ppm CO, and 100 ppmC1 of propene. The gas flow was controlled by rotameters and humidified by feeding nitrogen and oxygen through a water saturator at constant temperature.
2.2 Sampling Method and Determination of Blank Value
The gas stream leaving the reactor was quenched by a flow of deionized water. The resulting gas-liquid mixture was further cooled and fed through an absorption apparatus acc. to the German norm DIN 2462 which consists of two porous glass frits in series to collect both gases and aerosols quantitatively. From the bottom of the absorption apparatus, the solvent was recirculated by a peristaltic pump to the quenching point immediately after the reactor. Therefore, vanadium and tungsten accumulated in the liquid. The gas leaving the absorption apparatus flowed through three further gas washing bottles, of which the latter two were placed in a cooling bath at 10 °C to condense water vapors before the volumetric gas flow was measured in a gas meter.
After a measurement period, usually 24 h, the absorption liquid was collected, and the gas lines as well as the adsorption apparatus were washed first with 2 M NaOH and then with water while heating the setup to around 80 °C with a hot air blower to completely dissolve the well-observable deposits at the apparatus walls. All the liquid samples (collected adsorption liquid, NaOH solutions, washing water, and the content of the gas washing bottles) were analyzed by ICP-OES to determine the vanadium and tungsten content in these liquid samples.
In intermittent operation, the setup was cooled down after 24 h to collect the sampling liquids as described above. In continuous operation, the reactor with the catalyst was kept at the desired temperature and only the sampling tube at the outlet at the reactor was removed during 1 h to collect the sampling liquids.
To minimize the background or blank value of vanadium in the setup, the setup was cleaned between experimental runs by a thorough cleaning procedure involving washing with hot NaOH (especially the tubing and reactor walls) and hot concentrated H2SO4 (especially the frits) to remove efficiently solid deposits at the walls or small catalyst particles on the frits. The background or blank value of the setup was determined by conducting the measurement procedure described above, but with an inert piece of cordierite monolith in the reactor.
3 Results and Discussion
3.1 Limit of Detection
By adding the contents in the liquid samples and considering the gas flows through the setup, the vanadium, titanium, and tungsten content in the gas leaving the reactor could be determined.
The limits of detection (LoD) of these elements determined by repetitive measurements of standards by ICP-OES were 0.004 μg V/mLliquid sample for vanadium, 0.02 μg W/mLliquid sample for tungsten, and 0.002 μg Ti/mLliquid sample for titanium. Based on the total volume of the liquid samples (ca. 250 mL) and a typical gas flow of 13.9 m3 at STP in 24 h, the limit of detection of the sampling method with respect to the vanadium concentration would be (250 mL ∙ 0.004 μg V/mLliquid sample/13.9 m3 =) 0.07 μg/m3 at STP for vanadium and 0.35 μg/m3 at STP for tungsten. For the practical application in this study, LoD of the sampling method of 0.2 μg/m3 at STP for vanadium and 1.0 μg/m3 at STP for tungsten were chosen to consider uncertainties in the LoD of the ICP-OES.
3.2 Determination of Blank Values
For the determination of the background or blank value for measuring the V emissions with this setup and the sampling method, the sampling procedure was conducted several times at different temperatures using an inert cordierite honeycomb instead of a catalyst. The cleaning procedure (washing the reactor with hot NaOH, later additionally washing the frits with H2SO4) between the measurements was optimized. As shown in Fig. 2, the blank values increased with operation temperature and varied from below detection limit (<0.2 μg/m3 V at STP) at 500 °C to around 1 μg/m3 V at STP at 650 °C. Further, it could be observed that additional washing with H2SO4 instead of using hot NaOH only decreased the blank value by about half. Both findings supported the hypothesis that the blank value was dominated by deposits from previous experiments, which emphasizes the importance of very thorough cleaning of all surfaces involved. Therefore, for data points from experiments at 600 °C and below applying the cleaning with NaOH only, the upper limit of the 95 % range of the blank values determined at 600 °C was used as a blank value (0.5 μg/m3 V at STP). For 650 °C, the upper limit of the 95 % range of the blank values determined at 650 °C was used (1.0 μg/m3 V at STP); for data points at 625 °C, an interpolation was conducted resulting in a blank value of 0.75 μg/m3 V at STP.
3.3 Vanadium Emissions from the Catalyst at Elevated Temperatures
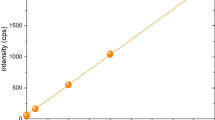
In a first series of experiments, the V emission at 600 °C during 24 h was determined in repeated experiments using a fresh catalyst sample each time to evaluate the reproducibility. As can be seen in Fig. 3, the values exceed the blank value by about 1 order of magnitude and lie in a range of ±19 % around 6.4 μg/m3 V at STP. As some issues with the temperature control were observed during these experiments and given the strong temperature dependence of the V emission (see next paragraph), one can expect that optimal temperature control might even allow further improvement of the reproducibility.
V emissions (measured minus blank) at 600 °C averaged during 24 h: repeated measurements to determine the reproducibility. If 95 % probability is calculated, the range lies at 6.4 ± 3 μg/m3 V at STP. The shown blank value is the upper limit of the 95 % probability range determined at 600 °C for cleaning with NaOH only
In a further series of experiments, the V emissions from one sample during 24 h were measured at different temperatures (Fig. 4). All measured values are significantly higher than the blank values applied at the respective temperatures. As expected when considering the increase of the vapor pressure, the emissions of V increase non-linearly with temperature and range from 2.4 μg/m3 V at STP at 575 °C to 33.2 μg/m3 V at STP at 650 °C.
V emissions (measured minus blank) at different temperatures averaged during 24 h. The shown blank values are the upper limit of the 95 % probability range determined for cleaning with NaOH only at 600 and 650 °C, respectively (625 °C: interpolation). The error bars have been determined at 600 °C (see Fig. 3)
The V emissions from the same sample of the commercial SCR catalyst were measured at 600 °C for ten times 24 h with intermediate cooling (so-called intermittent operation) and for three times 24 h in continuous operation without cooling to investigate whether the vanadium loss is stable under elevated, non-standard temperatures. As can be seen in Fig. 5, there are no significant differences between intermittent and continuous operation, i.e., it is concluded that the operation time at a certain temperature was dominant while heating and cooling did not seem to have an influence. From the time series, it can be seen that the V emissions increased during the first five times of 24-h operation to a value around 25–30 μg/m3 V at STP and then stayed constant, i.e., about fourfold of the average emission of the first 24 h was reached under long-term conditions. After 240 h, the vanadium content was decreased by less than 0.1 % of the initial vanadium content, while the specific surface decreased from initially 60 to 25 m2/g. This sintering is a drastic thermal effect, which caused a very strong deactivation of SCR activity.
3.4 Tungsten Emissions from the Catalyst at Elevated Temperatures
Beside the vanadium, also tungsten emissions were measured. As shown in Fig. 6, the tungsten emissions were about 20 to 30 times higher than the vanadium emissions. Like those of vanadium, they increased non-linearly from 30 μg/m3 W at STP at 575 °C to around 1200 μg/m3 W at STP at 650 °C. With respect to the tungsten evolution over time, the emissions of W during a series of 24-h operations with intermediate cooling were measured along with the V emissions shown in Fig. 5. Like vanadium, the tungsten emissions increased to a four times higher level (from 80 μg/m3 W at STP in the first 24 h to around 350 μg/m3 W at STP) during the first 100 h before stabilizing on the higher level (data not shown). The similarity between the behavior of tungsten and vanadium with respect to both temperature and operation time at a high temperature indicates that both elements are involved in a similar mechanism of formation of volatile hydroxides.
4 Conclusions
A liquid-quench-based experimental method was developed for the determination of even minimal vanadium and tungsten emissions from SCR catalysts by ICP-OES in the laboratory. A low limit of detection based on accumulation during long measurement times allows for indicative measurements even in the low-concentration range. The procedure requires a very thorough cleaning of the setup between the experiments to minimize residual vanadium deposits on surfaces and to allow for blank values significantly below the emission values. However, the increase of blanks with temperature could not be avoided and consequently this method cannot be recommended as a standard procedure in common analytical chemistry.
The method was applied to a commercial EuroV SCR catalyst (V2O5/WO3-TiO2) with normal operation temperatures below 450 °C, which was on purpose operated at out-of-spec temperatures above 550 °C in the laboratory. It was observed that the emissions of vanadium exponentially increase with temperature but the absolute concentrations if calculated to gas phase are very small and still below the legal limits (50 μg V/m3) of health organizations such as NIOSH [10]. In the presence of water, hydroxides of both elements are formed.
Further, it could be shown that with heating up of the same catalyst sample for 100 h, the emissions of both metals increase before reaching a stable level that could be up to four times higher than the emission level in the first 24 h. It is clearly shown that at elevated temperatures, initial heavy thermal damage of the catalyst occurred.
With the chosen ICP-OES analysis method, it was not possible to determine the type of vanadium compound emitted, i.e., the data represent the sum of all V compounds.
The experimental method determined the vanadium loss from the catalyst, but the presented values are not necessarily identical to the tailpipe emissions as the experiments showed that solid deposits can be easily formed at surfaces inside the after-treatment system.
Ongoing work in our laboratory focuses on sorbent-based methods for the determination of vanadium and tungsten emissions.
References
Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide, IARC Monograph on the Evaluation of Carcinogenic Risks to Humans, Vol. 86, Lyon, France (2006).
Mamane, Y., Pirrone, N.: In: Nriagu, J.O. (ed.) Vanadium in the environment, part 1: chemistry and biochemistry, pp. 37–71. John Wiley & Sons, New York (1998)
Immissionswerte für Vanadium und seine Verbindungen, Bericht des Unterausschusses “Wirkungsfragen” des Länderausschusses für Immissionsschutz, Herausgeber: Landeanstalt für Umweltschutz Baden-Württemberg.
Soldi, T., Riolo, C., Alberti, G., Gallorini, M., Peloso, G.F.: Sci. Total Environ. 181, 45–50 (1996)
Valiulis, D., Ceburnis, D., Sakalys, J., Kvietkus, K.: Atmos. Environ. 36, 6001–6014 (2002)
Hu, S., Herner, J.D., Shafer, M., Robertson, W., Schauer, J.J., Dwyer, H., Collins, J., Huai, T., Ayala, A.: Atmos. Environ. 43, 2950–2959 (2009)
Lough, G.C., Schauer, J.J., Park, J.S., Shafer, M.M., DeMinter, J.T., Weinstein, J.P.: Environ. Sci. Technol. 39, 826–836 (2005)
Shafer, M.M., Toner, B.M., Overdier, J.T., Schauer, J.J., Fakra, S.C., Hu, S., Herner, J.D., Ayala, A.: Environ. Sci. Technol. 46, 189–195 (2012)
Chapman, D.M.: Appl. Catal. A 392, 143–150 (2011)
National Institute for Occupational Safety and Health homepage: pocket guide for hazardous materials, http://www.cdc.gov/niosh/npg/npgd0654.html.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schildhauer, T.J., Elsener, M., Moser, J. et al. Measurement of Vanadium Emissions from SCR Catalysts by ICP-OES: Method Development and First Results. Emiss. Control Sci. Technol. 1, 292–297 (2015). https://doi.org/10.1007/s40825-015-0023-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-015-0023-x