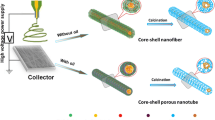
Abstract
Recent progress in nanoscience and nanotechnology creates new opportunities in the design of novel SnO2 nanomaterials for photocatalysis and photoelectrochemical. Herein, we firstly highlight a facile method to prepare three-dimensional porous networks of ultra-long SnO2 nanotubes through the single capillary electrospinning technique. Compared with the traditional SnO2 nanofibers, the as-obtained three-dimensional porous networks show enhancement of photocurrent and photocatalytic activity, which could be ascribed to its improved light-harvesting efficiency and high separation efficiency of photogenerated electron–hole pairs. Besides, the synthesis route delivered three-dimensional sheets on the basis of interwoven nanofibrous networks, which can be readily recycled for the desirable circular application of a potent photocatalyst system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It has been widely demonstrated that semiconducting materials are of great promise for energy and environmental applications such as photocatalytic water splitting for hydrogen production, dye-sensitized solar cells, and photocatalytic remediation of harmful organics from air and water [1–4]. As a typical n-type semiconductor, SnO2 has received great attention because of its excellent stability, nontoxicity, low-cost, and excellent optical electricity properties [5]. Especially, the exploitations of SnO2 for photocatalytic water splitting and photocatalytic oxidation of organic wastes have been hot topics because of its high reduction potential and low oxidation potential [6–8].
However, the practical performance of bulk SnO2 is far from the ideal case which has been limited by several factors such as short lifetime of the excited-state carrier (10−12 s), poor oxygen evolution reaction kinetics, and short hole diffusion length (2–4 nm) [9–15]. As we all know, the separation rate of photoinduced surface and volume charge carriers in a photocatalyst can be significantly increased by reducing its size to the appropriate nanoscale level and, thus, the photocatalytic activity can be enhanced. Therefore, considerable effort has been taken to synthesize photocatalysts with a small size so as to achieve high activities. However, a new disadvantage arises naturally: the recycling of the photocatalysts hinders their applications due to their small size. This has led to an unfavorable balance between the reduced charge recombination and a negative impact on recycling. Therefore, it is highly challenging but desirable to develop a direct effective approach for fabricating a new type of nanostructured photocatalyst with efficient electron–hole utilization, high specific surface areas, and favorable recycling characteristics.
Notably, electrospinning technique provides an effective approach to fabricate the three-dimensional porous supports with large surface area. In particular, it has been demonstrated successfully by different groups including ours that the three-dimensional structure composed of one-dimensional (1D) nanofibers or nanotubes catalysts with large length-to-diameter ratio can allow them to be readily separated from fluid by sedimentation [16–21]. Moreover, it is known that the structure and morphology also have a strong effect on the physical and chemical properties of photocatalysts, especially on the photocatalytic activities. Among various morphologies, hollow structures have attracted immense attention for their evidently improved performances over particles in photocatalysis and other applications. The hollow structures, especially those with tubular structures, have many useful features: (i) high surface-to-volume ratio enables it to adsorb a large amount of chemicals, (ii) hollow multi-channeled structures makes it convenient for mass transfer, (iii) the unique structure makes better use of light through multiple reflections within its hollow space.
Herein, we reported a successful attempt for the fabrication of the SnO2 nanotubes by the single capillary electrospinning technique. The investigation of photocatalytic ability indicated that the as-prepared composites exhibited high photocatalytic activity in the decomposition of Methyl Orange (MO). Also, free-standing sheets were readily delivered using the current processing routes, which enables circular exploitation of the materials as potent environmental catalysts.
2 Experimental Section
2.1 Fabrication of SnO2 Nanotubes and SnO2 Nanofibers
The fabrication of SnO2 NTs is schematically indicated in Scheme 1. To obtain nanotubes, two kinds of precursor solutions were prepared. Firstly, certain amounts of tin dichloride dihydrate (SnCl2·2H2O, Tianjin. Chemical Corp., China) were dissolved in a mixture of 2.2 g ethanol and 2.2 g N,N-dimethyl formamide by magnetic stirring for 1 h at room temperature. Secondly, 0.4 g polyvinyl pyrrolidone (PVP, Sigma Aldrich, Mw 1 300 000) was added to the resultant solution and vigorously stirred for 3 h at room temperature. The weight ratio of PVP to SnCl2·2H2O was 1.78. A high voltage of 20 kV was supplied by a direct-current power supply and the feeding rate for the precursor solution was adjusted to a constant rate of 0.3 mL h−1 using a syringe pump. A piece of aluminum foil was placed at 15 cm below the tip of the needle to collect the as-spun nanofibers. The process was carried out in air at room temperature. For the following thermolysis process, the as-spun nanofibers were placed in a muffle furnace and calcined at 600 °C in air for 3 h with a heating rate of 2 °C min−1, to remove PVP and obtain SnO2 nanotubes. By tuning the weight ratios of PVP to SnCl2·2H2O to 0.73, the samples of SnO2 nanofibers denoted as SnO2 NFs were fabricated.
2.2 Characterization
The morphologies of the as-prepared nanofibers were observed by scanning electron microscopy (FESEM, JSM-7500F) at an accelerating voltage of 20 kV, transmission electron microscopy (TEM; FEI Tecnai G2 F20) at an accelerating voltage of 300 kV. X-ray diffraction (XRD) was carried out with the 2θ range from 20 to 80° at a scan rate of 1° min−1 using a D/max 2500 XRD diffractometer (Rigaku) with Cu Kα radiation (0.1541 nm). The Brunauer-Emmett-Teller (BET)-specific surface area determination was performed by N2 gas adsorption using an America Micromeritics ASAP 2010 surface analytical instrument.
2.3 Photoelectrochemical Experiment
Photoelectrochemical measurements were performed using the conventional three electrode setup connected to an electrochemical station (CH Instrument 660C, Shanghai Chenhua, China). The setup had SnO2 NFs/FTO, and SnO2 NTs/FTO (effective area was 1 cm2, effective amount was 0.01 g) as working electrodes, and a Pt wire and an Ag/AgCl (saturated KCl) electrode were used as the counter electrode and reference electrode, respectively. The electrolyte was 0.5 M Na2SO4 aqueous solution. A 50 W high-pressure mercury lamp with main emission wavelength of 313 nm was used as the visible light source. The photocurrent response spectroscopy was carried out at a constant potential of +0.6 V to the working photoanode.
2.4 Photocatalytic Test
The photocatalytic activities were evaluated by the decomposition of MO under UV light. The photoreactor was designed with an internal light source surrounded by a quartz jacket (50 W high-pressure mercury lamp with output light intensity of 1,200 mW cm−2 and main emission wavelength of 313 nm), where a 100 mL of the model dye (MO) solution with an initial concentration of 10 mg L−1 in the presence of solid catalyst (0.01 g), respectively. The solution was stirred in the dark for 30 min to obtain a good dispersion and reach adsorption–desorption equilibrium between the organic molecules and the catalyst surface. Decreases in the concentrations of dyes were analyzed by a Cary 500 UV–Vis-NIR spectrophotometer at λ = 553 nm. At given intervals of illumination, the samples (3 mL) of the reaction solution were taken out and centrifuged. Finally, the filtrates were analyzed. The fabrication process of SnO2 nanotube is illustrated in Fig. 1.
3 Results and Discussion
3.1 SEM of the As-Prepared Composite Nanofibers
The morphologies were examined by SEM and representative images are shown with the as-prepared SnO2 NTs. It could be seen from Fig. 2a that a free-standing three-dimensional network structure was composed of a large quantity of randomly deposited nanotubes. Figure 2b showed that the individual fiber had a high aspect ratio and a smooth surface, and the lengths of these randomly oriented nanotubes could reach several micrometers, and the diameters of those nanotubes ranged from 120 to 200 nm. Furthermore, Fig. 2c, d showed the high-magnification SEM image of Fig. 2b. It was observed that the SnO2 NTs had a smooth and clean surface with inner diameter of about 260 nm. In order to determine the chemical composition of the core/shell nanotube, EDX spectra (Fig. S1 in ESM) of SnO2 NTs in conjunction with SEM indicated that the SnO2 NTs were composed of Sn, O, and C, and the Pt peak came from the conductive coating when operating the SEM. It was worth pointing out that the atomic ratio of Sn to O was approximated to 1:2, which was close to the theoretical value.
3.2 TEM of the As-Prepared Composite Nanofibers
In order to obtain more detailed information about the morphology and crystalline structure of the SnO2 NTs, the TEM and high resolution TEM (HRTEM) measurements were carried out. Figure 3a showed the typical TEM of the SnO2 NTs. It’s interesting to note that the SnO2 NTs were composed of nanoparticles, and each nanoparticle attached to several other nanoparticles. Furthermore, Fig. 3b showed the high-magnification TEM image of Fig. 3a. It was observed that the inner diameter of SnO2 NTs was about 260 nm and the wall thickness was about 45 nm. In Fig. 3c, the lattice spacing is 0.335 nm, which could be assigned to the plane [110] of SnO2 [22].
3.3 XRD Patterns
XRD analyzes were conducted to assess the structure and phase purity of the SnO2. As observed in Fig. 4, for pure SnO2 NTs and SnO2 NFs, ten reflection peaks appeared at 2θ = 26.59° (110), 33.88° (101), 38.98° (200), 51.78° (211), 54.46° (220), 53.0° (422), 57.67° (002), 62.02° (310), 71.3° (202), 78.2° (321), which were attributed to the tetragonal rutile SnO2 structure (space group P42/mnm, a0 = b0 = 4.737 Å, c0 = 3.186 Å, JCPDS, No. 88-0287). And, there was no evident diffraction peak attributed to Sn and SnO secondary phases, suggesting a relatively pure SnO2 phase could be obtained after calcination at 600 °C in air. Moreover, the average grain sizes of the products were calculated from SnO2 (110) peak by applying the Debye–Scherrer formula. It could be seen that the average grain size of SnO2 NPs was about 10 nm for both SnO2 NTs and SnO2 NFs, which was consistent with TEM observations.
3.4 Photocatalytic Activity
To demonstrate the photoactivity of the as-obtained SnO2 NTs for the degradation of organic pollutants, we carried out the experiments of the photocatalytic degradation of MO as a test reaction. Furthermore, in the comparative experiments, the pure SnO2 NFs were used as a photocatalytic reference to understand the photocatalytic activity of the SnO2 NTs. As observed in Fig. 5a, b, the control experiments were performed under different conditions: (1) in the presence of photocatalysts (0.01 g) but in the dark; (2) with UV irradiation but in the absence of the photocatalysts. These control experiments revealed that there was no appreciable degradation of MO over the SnO2 NTs in the absence of UV light irradiation, indicating that the adsorption–desorption equilibrium of MO in the dark was established within 30 min. And, there was no appreciable degradation of MO in the absence of photocatalysts. The change in absorption spectra of MO aqueous solution showed the change of its concentration. The initial concentration (C0), the final concentration (C), and the degradation rate (D %) had a mathematical expression as follows
As shown in Fig. 6, after UV light irradiation for 40 min, the degradation efficiency of MO was about 50 and 88 % for SnO2 NFs and SnO2 NTs, respectively. Obviously, the SnO2 NTs showed much higher photocatalytic activities than that of the SnO2 NFs. What’s more, for a better comparison of the photocatalytic efficiency of the SnO2 NFs and SnO2 NTs, the kinetic analysis of degradation of MO was discussed. The kinetic linear simulation curves of the photocatalytic degradation of MO over the above catalysts showed that the above degradation reactions followed a Langmuir–Hinshelwood apparent first-order kinetics model due to the low initial concentrations of the reactants. The explanation is described below:
where r is the degradation rate of the reactant (mg L min−1), C is the concentration of the reactant (mg L−1), t is the UV light irradiation time, k is the reaction rate constant (mg L min−1)), and K is the adsorption coefficient of the reactant (L mg−1). When the initial concentration (C0) is very low (C0 = 10 mg L−1 for MO in the present experiment), Eq. (2) can be simplified to an apparent first-order model:
where kapp is the apparent first-order rate constant (min−1). The determined kapp values for different catalysts were summarized in Fig. S3 in ESM. The photocatalytic reactivity order was SnO2 NTs > SnO2 NFs, which was well-consistent with the activity studied above.
Moreover, cycling uses as well as maintaining high photocatalytic activity was a critical issue for long-term use in practical applications of the catalyst. Consequently, two factors were needed to be considered: (i) the stability of the catalyst to maintain its high activity over time. It was known that the photocorrosion or photodissolution of catalyst might occur on the photocatalyst surface during the photocatalytic reaction. To test the stability of SnO2 NTs, we reused the catalyst for five times. As shown in Fig. 7a, each experiment was carried out under identical conditions in which a 100.0 mL of the model dye (MO) solution with an initial concentration of 10 mg L−1 in the presence of solid catalyst (0.01 g), and the photocatalytic activity of the SnO2 NTs remained almost unchanged in the recycling reactions. Figure 7b showed the SEM images of the SnO2 NTs after the fifth catalytic reaction. It could be seen that the nanostructure of SnO2 NTs was still very complete, clearly indicating the stability. (ii) The ease with which the catalyst could be recycled from solution. The samples were of one-dimensional nanotubes morphology and could be easily separated from an aqueous suspension. The photograph in Fig. 7 showed that the samples could be easily separated from the solution by sedimentation, probably due to the large length-to-diameter ratio of the one-dimensional SnO2 NTs. It was indicated that the SnO2 NTs displayed an efficient photoactivity for the degradation of organic pollutants under UV light irradiation and could easily be recycled for reuse.
3.5 Postulated Photocatalytic Mechanism of the SnO2 NTs
It was well known that the photocatalytic activity was mainly governed by phase structure, adsorption ability, and separation efficiency of photogenerated electrons and holes. As could be seen from the XRD analysis, the crystal phase structure of the SnO2 NTs was nearly similar to that of SnO2 NFs. An adsorption experiment was performed to evaluate the adsorption ability of the SnO2 NFs and SnO2 NTs photocatalysts in the dark. As could be seen from Fig. 6, after equilibration in the dark for 30 min, 84 and 80 % of MO remained in the solution with SnO2 NFs and SnO2 NTs, respectively. Obviously, there were significant changes in the BET surface area (19.231 and 32.832 m2 g−1 for SnO2 NFs and SnO2 NTs). The enhancement of adsorption could be contributed to the increased surface area of the nanotubes. MO molecules could be adsorbed on the surface of SnO2 NTs until an adsorption–desorption equilibrium was reached. Compared with SnO2 NFs, the enhanced adsorptivity was a good supplement for the high photocatalytic activity of the SnO2 NTs photocatalyst.
Besides the high adsorptivity, the significant enhancement of photocatalytic activity was mainly due to the high efficiency of charge separation. According to traditional semiconductor theory shown in Scheme 1, SnO2 could be excited by UV light and produced photogenerated electron–hole pairs, showing photocatalytic activity. For both SnO2 NFs and SnO2 NTs, the mechanism for the photocatalytic degradation of MO in our experiment was proposed as follows (1–7):
As we know, the structure and morphology also have a strong effect on the efficiency of charge separation. Compared with SnO2 NFs, the unique nanotube structure makes better use of light through multiple reflections within its hollow space. Through enhancing the efficiency of light absorbance, the number of photoexcited charge carriers will be increased. What’s more, the underlying but probably more important advantages are the shorter bulk diffusion length produced by nanotubes with ultrathin thickness, and the hollow multi-channeled structure makes it convenient for mass transfers, which plays an important role in prolonging the lifetime of charge carriers and improve the quantum yield.
Furthermore, the recombination rate of photoexcited charge carriers can be characterized by detecting the photocurrents generated [23–27]. Photoelectrochemical measurements were often used to qualitatively study the excitation and transfer of photogenerated charge carriers in photocatalysts. The transient photocurrent responses of the SnO2 NFs and SnO2 NTs were recorded for three on–off cycles under UV light irradiation and plotted in Fig. 8. As expected, SnO2 NTs show the higher photocurrent intensity than SnO2 NFs. Thus, the sample SnO2 NTs may achieve the more effective charge separation, which is consistent with the photocatalytic activity measurements and our above discussions.
4 Conclusions
In summary, we describe herein an effective route to synthesize three-dimensional porous networks of ultra-long SnO2 nanotubes through the single capillary electrospinning technique. Compared with the traditional SnO2 nanofibers, the as-obtained three-dimensional porous networks show enhancement of photocurrent and photocatalytic activity, which could be ascribed to its improved light-harvesting efficiency and electron transport ability along the in-plane direction, and increased lifetime of photoexcited charge carriers. Besides, the synthesis route delivered three-dimensional sheets on the basis of interwoven nanofibrous networks, which can be readily recycled for the desirable circular application of a potent photocatalyst system. Notably, the free-standing 3D nanotubes network structure could improve photocatalyst’s performance of separation and reuse. Also, it is expected that the SnO2 NTs network will promote their industrial application as clean energy materials.
References
B. O’Regan, M. Gratzel, A low-cost high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346), 737–740 (1991). doi:10.1038/353737a0
M. Batzill, Fundamental aspects of surface engineering of transition metal oxide photocatalysts. Energy Environ. Sci. 4(1), 3275–3286 (2011). doi:10.1039/c1ee01577j
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Environmental applications of semiconductor photocatalysis. Chem. Rev. 95(1), 69–96 (1995). doi:10.1021/cr00033a004
S.U. Khan, M.M. Al-Shahry, W.B. Ingler Jr, Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297(5590), 2243–2245 (2002). doi:10.1126/science.1075035
C. Zhu, P. Wang, L. Wang, L. Han, S. Dong, Facile synthesis of two-dimensional graphene/SnO2/Pt ternary hybrid nanomaterials and their catalytic properties. Nanoscale 3(1), 4376–4382 (2011). doi:10.1039/c1nr10634a
Y. Liu, Y. Jiao, B. Yin, S. Zhang, F. Qu, X. Wu, Hierarchical semiconductor oxide photocatalyst: a case of the SnO2 microflower. Nano-Micro Lett. 5(4), 234–241 (2013). doi:10.1007/BF03353754
M.H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo, P. Yang, Room-temperature ultraviolet nanowire nanolasers. Science 292(5523), 1897–1899 (2001). doi:10.1126/science.1060367
H. Kind, H. Yan, B. Messer, M. Law, P. Yang, Nanowire ultraviolet photodetection and optical switches. Adv. Mater. 14(2), 158–160 (2002). doi:10.1002/1521-4095(20020116)14:2<158:AID-ADMA158>3.0.CO;2-W
N. Chadwick, S.N. Sathasivam, A. Kafizas, S.M. Bawaked, A.Y. Obaid, S. Al-Thabaiti, S.N. Basahel, I.P. Parkina, C.J. Carmalt, Combinatorial aerosol assisted chemical vapour deposition of a photocatalytic mixed SnO2/TiO2 thin film. J. Mater. Chem. A 2(1), 5108–5116 (2014). doi:10.1039/c4ta00545g
H. Wang, S. Kalytchuk, H. Yang, L. He, C. Hu, W.Y. Teohc, A.L. Rogach, Hierarchical growth of SnO2 nanostructured films on FTO substrates: structural defects induced by Sn(II) self-doping and their effects on optical and photoelectrochemical properties. Nanoscale 6(11), 6084–6091 (2014). doi:10.1039/c4nr00672k
Q. Gu, J. Long, H. Zhuang, C. Zhang, Y. Zhou, X. Wang, Ternary Pt/SnOx/TiO2 photocatalysts for hydrogen production: consequence of Pt sites for synergy of dual co-catalysts. Phys. Chem. Chem. Phys. 16(1), 12521–12534 (2014). doi:10.1039/c4cp01496k
B. Jia, W. Jia, X. Wu, F. Qu, Hierarchical porous SnO2 microflowers photocatalyst. Sci. Adv. Mater. 4(11), 1127–1133 (2012). doi:10.1166/sam.2012.1404
B. Jia, W. Jia, Y. Ma, X. Wu, F. Qu, SnO2 core-shell microspheres with excellent photocatalytic properties. Sci. Adv. Mater. 4(7), 702–707 (2012). doi:10.1166/sam.2012.1341
Y. Liu, Y. Jiao, Z. Zhang, F. Qu, A. Umar, X. Wu, Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor and supercapacitor applications. ACS Appl. Mater. Interfaces 6(3), 2174–2184 (2014). doi:10.1021/am405301v
B. Jia, W. Jia, F. Qu, X. Wu, General strategy for self-assembly of mesoporous SnO2 nanospheres and their applications in water purification. RSC Adv. 3(1), 12140–12148 (2013). doi:10.1039/c3ra41638k
P. Zhang, C. Shao, Z. Zhang, M. Zhang, J. Mu, Z. Guo, Y. Liu, TiO2@carbon core/shell nanofibers: Controllable preparation and enhanced visible photocatalytic properties. Nanoscale 3(1), 2943–2949 (2011). doi:10.1039/c1nr10269a
P. Zhang, C. Shao, Z. Zhang, M. Zhang, J. Mu, Z. Guo, Y. Liu, Core/shell nanofibers of TiO2@carbon embedded by Ag nanoparticles with enhanced visible photocatalytic activity. J. Mater. Chem. 21(1), 17746–17753 (2011). doi:10.1039/c1jm12965a
P. Zhang, C. Shao, M. Zhang, Z. Guo, J. Mu, Z. Zhang, X. Zhang, P. Liang, Y. Liu, Controllable synthesis of Zn2TiO4@carbon core/shell nanofibers with enhanced photocatalytic performance. J. Hazard. Mater. 229–230, 265–272 (2012). doi:10.1016/j.jhazmat.2012.05.102
P. Zhang, J. Zhang, J. Gong, Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 43(1), 4395–4422 (2014). doi:10.1039/c3cs60438a
H. Tang, C.M. Hessel, J. Wang, N. Yang, R. Yu, H. Zhao, D. Wang, Two-dimensional carbon leading to new photoconversion processes. Chem. Soc. Rev. 43(1), 4281–4499 (2014). doi:10.1039/c3cs60437c
P. Zhang, C. Shao, Z. Zhang, M. Zhang, J. Mu, Z. Guo, Y. Liu, In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 3(1), 3357–3363 (2011). doi:10.1039/c1nr10405e
X. Zhou, Z. Dai, S. Liu, J. Bao, Y. Guo, Ultra-uniform SnOx/carbon nanohybrids toward advanced lithium-ion battery anodes. Adv. Mater. 26(23), 3943–3949 (2014). doi:10.1002/adma.201400173
H. Zhang, X. Lv, Y. Li, Y. Wang, J. Li, P25-graphene composite as a high performance photocatalyst. ACS Nano 4(1), 380–386 (2010). doi:10.1021/nn901221k
C. Zhang, S. Yu, Nanoparticles meet electrospinning: recent advances and future prospects. Chem. Soc. Rev. 43(1), 4423–4448 (2014). doi:10.1039/c3cs60426h
T. Lv, L. Pan, X. Liu, T. Lu, G. Zhu, Z. Sun, Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite synthesized via microwave-assisted reaction. J. Alloy. Compd. 509(41), 10086–10091 (2011). doi:10.1016/j.jallcom.2011.08.045
Y. Wang, R. Shi, J. Lin, Y. Zhu, Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 4(2), 2922–2929 (2011). doi:10.1039/c0ee00825g
P. Russo, A. Hu, G. Compagnini, Synthesis, properties and potential applications of porous graphene: a review. Nano-Micro Lett. 5(4), 250–273 (2013). doi:10.1007/BF03353757
Acknowledgments
The present work is supported financially by the National Natural Science Foundation of China (Nos. 51001091, 111174256, 91233101) and the Fundamental Research Program from the Ministry of Science and Technology of China (No. 2014CB931704), and Project funded by China Postdoctoral Science Foundation(No. 2014M560602).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zhang, P., Wang, L., Zhang, X. et al. Three-dimensional Porous Networks of Ultra-long Electrospun SnO2 Nanotubes with High Photocatalytic Performance. Nano-Micro Lett. 7, 86–95 (2015). https://doi.org/10.1007/s40820-014-0022-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40820-014-0022-4