Abstract
Young children frequently report imaginary scary things in their bedrooms at night. This study examined the remembrances of 140 preschool children and 404 adults selecting either above, side, or below locations for a scary thing relative to their beds. The theoretical framework for this investigation posited that sexual-size dimorphism in Australopithecus afarensis, the presumed human ancestor in the Middle Pliocene, constrained sleeping site choice to mitigate predation. Smaller-bodied females nesting in trees would have anticipated predatory attacks from below, while male nesting on the ground would have anticipated attacks from their side. Such anticipation of nighttime attacks from below is present in many arboreal primates and might still persist as a cognitive relict in humans. In remembrances of nighttime fear, girls and women were predicted to select the below location and males the side location. Following interviews of children and adult questionnaires, multinomial log-linear analyses indicated statistically significant interactions (p < 0.001) of sex by location for the combined sample and each age class driven, in part, by larger frequencies of males selecting the side location and females selecting the below location. Data partitioning further revealed that males selected the side location at larger frequencies (p < 0.001) than the below location, whereas female selection of side and below locations did not differ significantly. While indicative of evolutionary persistence in cognitive appraisal of threat locations, the female hypothesis did not consider natural selection acting on assessment of nighttime terrestrial threats following the advent of early Homo in the Late Pliocene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation provides an explicit context impacting individual fitness that is amenable to experimental studies of antipredator behavior. The fearful behavior of young children in unfamiliar situations including darkness has been linked to child-adult attachment behavior by John Bowlby (1969/1982, p. 191) who stated that: “All in all, it is held, of the various suggestions advanced for the function of attachment behaviour, protection from predators seems by far the most likely.” The current study examines a feature of Bowlby’s evolutionary construct in which young children are fearful of something scary in their bedrooms at night in a manner that might reflect historical anticipation of predatory threats by human ancestors.
A prospective predatory threat to a perceiver includes its relative location and distance as part of the contextual features of the whole situation. As described by Chemero (2003, p. 184): “Affordances, I argue, are relations between particular aspects of animals and particular aspects of situations.” Along with the physical properties of the environment, the locations of anticipated threats can be considered “directional affordances” (see Sirkin et al., 2011, p. 167) as components of this overall relationship.
Scanning as a directional affordance is ubiquitous in species that routinely monitor the horizon or sky for potential predators (e.g., ground squirrels: Arenz & Leger, 1997; rats: Land, 2013; birds: Marler & Evans, 1996). Rabbits and ungulates with laterally directed eyes exhibit a horizontal “visual streak” with an increased density of retinal ganglion cells facilitating the detection of predators on the horizon (Rehkämper et al., 2000). The predominance of the visual streak in many prey species living in diverse habitats ranging from forests to savannas led Hughes (1977) to posit his “Terrain Theory” of predator detection.
Although other researchers have considered predation as an important component of human evolution (e.g., Baldwin, 2013; Barrett, 2005; Mobbs et al., 2015), Bowlby’s evolutionary construct provides a specific context for studying the locational aspects of the nighttime fear of young children that facilitates parental intervention such as holding and co-sleeping (see Miller & Commons, 2010). In related research considering the spatial properties of historical predation risk at night, Spörrle and Stich (2010) examined the preferred bed locations relative to doors and windows of adolescent and adult participants. Beds were positioned by participants in orientations relative to doors and windows that would facilitate detection of a potential aggressor. To explore whether there is a potential sex difference in the locational aspects of children’s nighttime fear, I review the sexual-size dimorphism of great apes and the presumed human ancestor Australopithecus afarensis in which body size would constrain sleeping site choice and assessment of the location of predatory threats at night.
Sexual-Size Dimorphism, Niche Partitioning, and Predation Risk
A seminal modeling paper by Schoener (1969) provided a cost-benefit evolutionary argument for a causal association of sexual-size dimorphism and niche partitioning by male and female Anolis lizards, a theoretical construct elaborated further by Slatkin (1984). In Greater Antilliean Anolis lizards, the larger males inhabit primarily the ground with smaller females inhabiting the tree trunks, or males the trunks and females the tree crowns (Butler et al., 2000). Such niche partitioning, also labeled “sexual dinichism” (see Susman et al.,1984, p. 149), is hypothesized as an evolutionary property of hominin evolution that might be revealed as a behavioral relict in children’s nighttime fear.
Sexual-size dimorphism can be characterized by a ratio, dividing the estimated average weight of heavier males by female weight, which yields a ratio of 1.07 to 1.08 for humans, 1.23 for common chimpanzees (Pan troglodytes), 1.36 for bonobos (P. paniscus), and 1.63 to 2.37 for gorillas (Leigh & Shea, 1995; Cameron, 2004; Garvin, 2012; also see Plavcan, 1994, p. 467 for computational argument). Female mountain gorillas (Gorilla beringei beringei) are observed to climb trees twice as often than much heavier silverback males (Schaller, 1963). In lowland gorillas (G. g. gorilla), sexual-size dimorphism constrains extractive foraging activity in trees. Remis (1995) reports that females are able to forage on lower weight-bearing branches than males.
The Middle-Pliocene hominin (Australopithecus afarensis) from the Afar region of northern Ethiopia, extending south in the East African Rift Valley to northern Tanzania, is generally accepted as a human ancestor (e.g., Villmoare et al., 2015; Wood & Lonergan, 2008). The larger estimated body mass of males (~ 45 kg) versus females (~ 29 kg) using a human-like scaling pattern (McHenry, 1992; McHenry & Coffing, 2000) yields a sexual-size dimorphism ratio of 1.55 (or 1.59 based on the comprehensive sample from Grabowski et al., 2015, p. 90) that approaches those of gorillas (cf. Gordon et al., 2008; Scott & Stroik, 2006). However, this general consensus of gorilla-like dimorphism has been challenged by Reno and Lovejoy (2015) who argue for a more moderate dimorphism. Other evidence of body size dimorphism is apparent in the presumed Au. afarensis footprints at Laetoli in northern Tanzania that support this contentious argument for considerable sexual dimorphism in body size similar to that of gorillas (Masao et al., 2016).
Sleeping in trees to mitigate nighttime predation has had a long history in primate evolution and most likely reflects the effect of natural selection acting on failure to select appropriate sleeping sites inaccessible to predators (Anderson, 1998; Fan & Jiang, 2008). An innate understanding that sleeping on the ground is hazardous is expressed by nearly all species of Old World monkeys. In southern India, bonnet macaques (Macaca radiata), Nilgiri langurs (Trachypithecus johnii), and Hanuman langurs (Semnopithecus entellus) choose sleeping sites on low weight-bearing branches near the edge of tree crowns to mitigate predation from heavier-bodied leopards (Panthera pardus) and pythons (Python molurus) that are agile climbers (Ramakrishnan & Coss, 2000, 2001).
Nest building in trees is evident in chimpanzees, gorillas, and orang-utans (cf. Pruetz et al., 2008; van Casteren et al., 2012). Koops et al. (2007, p. 409) posit that ground-nesting in chimpanzees (Pan troglodytes verus) in Guinea, West Africa, is a sex-linked behavior in which the heavier males construct all the elaborate ground nests. Chimpanzees from field sites with both low and high predation risk act as if they expect arboreal attacks at night because they tend to construct nests closer to the canopy edge that limits accessibility by leopards (Stewart & Preutz, 2013). While there are no reported observations of nighttime leopard predation on forest chimpanzees nesting in trees, daytime predation can be very high. For example, in the Taï National Park, Côte d’Ivoire, the mortality risk of leopard predation on forest chimpanzees is estimated to be 0.055 per year with an average likelihood of being killed within 18 years (Boesch, 1991). Therefore, sleeping off the ground by chimpanzees is prudent for survival in areas with high predation (cf. Koops et al., 2007, 2012; Pruetz et al., 2008). From a phylogenetic standpoint relevant to the current experiment, it is reasonable to argue that the last common ancestor of chimpanzees and humans constructed nests in trees to avoid predators at night (Pi, Veà, & Serrallonga, 1997).
Evolutionary Persistence of Behavioral Relicts
As the probable stem hominin living in a diversity of woodland and grassland habitats, the 3.9- to 3.0-million-year (Ma) period of stasis of identifiable Au. afarensis fossils (Kimbel, Johanson & Rak 1994; Ward, 2002) coincides with a consistent pattern of fluctuating wet and dry conditions driving changes in woodlands to grasslands in the East African paleoclimatic record (see Fig. 6 in deMenocal, 1995). The continuity of recognizable Au. afarensis fossils for ~ 0.9 Ma is suggestive of ecological patterns of stabilizing natural selection on adaptive morphology and behavior in the East African Rift Valley (Ward, 2002). This period of stabilizing selection would be followed by the progressive inclusion of meat and fat in the hominin diet by 2.6 Ma (de Heinzelin et al., 1999) that would energetically support brain enlargement (Leonard & Robertson, 1994; Pontzer et al., 2016).
The bauplan of Au. afarensis consists of a mixture of arboreal- and bipedal-based morphology with the arboreal features consisting of long arms, short legs, and curved fingers that would facilitate slow vertical climbing. In particular, the partial skeleton of the small female A.L. 288-1 “Lucy” has engendered extensive debates about her ability to climb trees effectively and walk with a human-like gate (cf. Latimer et al., 1987; Stern, 2002; Crompton, 2016; Melillo, 2016). This arboreal debate was partially settled when Lucy’s ~ 3.2 Ma fractured skeletal remains indicated that she died falling while climbing at considerable height in a tall tree (Kappelman et al., 2016).
In addition to sexual dimorphism in body size, there is suggestive skeletal evidence that males were less habitually arboreal than females. For example, the large, presumed male australopith KSD-VP-1/1 dated at ~ 3.6 Ma discovered at Woranso-Mille, Ethiopia (Haile-Selassie et al., 2010, p. 12,124; Melillo, 2016) exhibits a scapula with a moderately superiorly oriented glenoid fossa (obliquely tilted shoulder joint cavity) coupled with a more human-like laterally facing scapular spine. In contrast, the 3.3-Ma partial skeleton of a 3-year-old presumed female specimen DIK-1-1 exhibits a more gorilla-appearing scapula with a superiorly oriented spine and glenoid fossa (Alemseged et al., 2006). Lucy’s scapular fragments are insufficient for calculating spine orientation; albeit, they do show a superiorly oriented glenoid fossa in-between those of KSD-VP-1/1 and DIK-1-1 that overlaps the range of gorillas, but not humans (cf. Melillo, 2016, p. 129; Stern & Sussman, 1983). Stern and Sussman (1983, p. 284) argue that the superiorly orientated glenoid cavity is an adaptation for elevated positioning of the arms during climbing. If the superior orientation of the scapular spine of juvenile female DIK-1-1 was developmentally maintained in adult females as it appears to be in Lucy, it would be another sexually dimorphic trait to complement heavier male body mass for asserting that male and females differed in their degree of arboreality as proffered by Susman et al. (1984). Nevertheless, the youngest Hadar specimen A.L. 438-1 dated at ~ 3.0 Ma, and one of the largest males discovered, continues to display morphological traits useful for climbing, such as curved ulnae that would afford powerful forearm gripping for slow vertical climbing (Drapeau et al., 2005). Another arboreal feature of the DIK-1-1 juvenile is the deep and wide olecranon fossa of the distal humerus (Alemseged et al., 2006) that allows slightly greater elbow extension (for humans see Ndou, 2018, p. 91) useful in suspensory postures while climbing. As remnants of possible small-bodied females, the fossil ulnae A.L. 137-48a and A.L. 322-1 m from Hadar also exhibit deep and moderately deep olecranon fossae, respectively (Lovejoy et al., 1982).
Another facet of hypothesized male and female niche partitioning is reflected by dentition adapted to chewing softer and harder food items. Examination of the third premolar (P3) in a distribution of smaller- and larger-bodied Au. afarensis revealed sexual dimorphism indicated by larger tooth size and derived molarization of P3s in presumed males that would characterize ground-level foraging on low-quality plant foods requiring grinding (Leonard & Hegmon, 1987). Moreover, the more primitive morphology of female P3s is relatively stable over time suggestive of consistent arboreal foraging, whereas the males exhibit progressive molarization independent of tooth and body size that might indicate expanded terrestrial ranging.
I will now argue from a theoretical perspective that lighter-bodied female Au. afarensis typically constructed nests in trees, while heavier males built nests predominantly on the ground. Females and juveniles in night nests would not be safe from predation as leopards currently hunt primates in trees mostly at night (Isbell et al., 2018; Jenny & Zuberbühler, 2005). The earliest leopard fossil was discovered in the Laetoli beds of northern Tanzania dated 3.83 to 3.62 Ma in the same assemblage as the false sabertooth cat, Dinofelis petteri (Barry, 1987; Turner, 1990; Werdelin et al., 2014). Dinofelis petteri also appears at Hadar 3.5–3.0 Ma along with the dirk-toothed cat Megantereon spp., another likely primate predator found less frequently throughout eastern Africa (Boaz, Howell & McCossin, 1982; Werdelin & Lewis, 2005). The short tails of both cats (Christiansen, 2013; Werdelin & Lewis, 2001) would not be useful for arboreal balancing and high-speed maneuvering, which suggests that these cats were short-distance ambush hunters. Leopards typically ambush from cover, and this context might account for a predation incident on a hominin occurring much later in time at an Early Pleistocene site in Swartkrans, South Africa. As described by Brain (1970, 1981), the cranial vault of a large Paranthropus robustus exhibited two punctures that matched the spacing of the lower canines of a leopard positioned on the cranium for carcass dragging to prevent stealing.
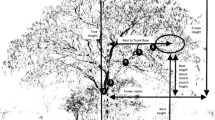
Due to nighttime threats from these large saber-cats and early leopards and lions (Panthera cf. leo, see Barry, 1987), it is reasonable to theorize that much like male gorillas, solitary Au. afarensis males, mostly constructed ground-based nests near groups of trees with nesting females that also provided the opportunity for emergency tree climbing. Because of the availability of outcrops of eroded boulders from earlier lava flows near groves of trees within the East African Rift Valley (see King & Bailey, 2006; Bailey & King, 2010), it is possible that solitary males or bachelor groups found boulder crevices and other defensive areas like fault scarps useful for protection against nighttime predators (for baboons, see Cowlishaw, 1997). In either setting, the spatial context for predation would have been that nesting females in trees (Fig. 1) were vulnerable to nighttime attacks by leopards ascending from underneath them, while ground-nesting males were vulnerable to sideways attacks by leopards and other large, nonarboreal cats (see Treves & Palmqvist, 2007). Due to its long history extending well into Miocene times, natural selection on appraising microhabitats for their refuge affordances in the human lineage would have likely included anticipation of the directional properties of potential predatory attacks.
What is the likelihood that arboreal tendencies continue to be expressed in modern humans? The small bodies of pygmy hunter-gatherers and their high humerofemoral body proportions arguably reflect natural selection for safe, routine foraging in trees. In one Philippine population, for example, routine tree climbing has remodeled the length of calf muscles facilitating extreme dorsiflexion for pedal traction during slow vertical climbing (Venkataramana, Kraft & Dominy, 2013, p. 1238). Genetic factors as well as sex hormones can also play a role in shaping joint hypermobility. Qvindesland and Jónsson (1999) report in Islandic children that 40.5% of girls and 12.9% of boys exhibit joint hypermobility and, in one African population, females of all ages are more hypermobile than same-age male cohorts (Beighton et al., 1973). In other developmental studies, young girls typically exhibit greater articular laxity than boys enhancing their range of motion, notably in ankle plantarflexion (Alanen et al., 2001; Soucie et al., 2011) that is also apparent in female ballet dancers on point. In Au. afarensis, the putative greater degree of plantarflexion would extend the reach of the foot for seeking branch support, a behavioral property thought to have been enhanced by the downward orientation of the proximal edge of distal articular facet (malleolar articular surface) of Lucy’s fibula. The horizontal orientation of the larger articular facets of presumed male Au afarensis falls within human range (Stern & Susman, 1983, p. 305), thus inspiring the construct of sexual dinichism in degree of arboreality (Susman et al., 1984, p. 149); albeit, Lucy’s estimated ankle range of motion also includes extreme dorsiflexion that would have been useful for slow vertical climbing (cf. Damiano, 2015; Latimer et al., 1987). Although restricted to a single population examined, Japanese men and women do differ reliably at the multivariate level in the orientation of the ankle malleolar articular surface (Sacragit & Ikeda, 1995) that might characterize a faint relict of arboreal niche partitioning.
There is considerable individual variation in modern human female and male joint morphology that could be explained by prolonged relaxed selection on climbing leading to genetic drift. In light of the aforementioned Au. afarensis deep olecranon fossae and arm extension, Rogers (1999) reports that the distal humerus of modern females has a deep, oval-shaped olecranon fossa compared with a shallow, more triangular fossa of males. However, other studies report that this sex difference in elbow structure is highly variable and is only predictive of sex in a multivariate combination with other elbow characteristics (Tallman & Blanton, 2020).
While continued sexual dimorphism in modern human body size and joint articular characteristics lend support to my argument for arboreal niche partitioning deep in the human lineage, are there perceptual and cognitive attributes under relaxed selection for assessing historical threats still persisting from Pliocene times? The current experiment on the relaxed selection of niche partitioning was inspired, in part, by evidence that California ground squirrels (Otospermophilus beecheyi) from snake-free and snake-rare habitats can innately discriminate their ancestral rattlesnake and gopher snake predators for a period spanning more than 300,000 years (Coss & Goldthwaite, 1995; Coss, 1991a, 1993, 1999). Snake recognition does disintegrate between this time frame and 3 Ma as evidence by the loss of snake recognition by Arctic ground squirrels (Spermophilus parryii) (Goldthwaite, Coss & Owings 1990). If viewed in the context of species generation times that introduce mutations altering adaptive patterns of interneural connectivity, ground squirrels have a generation time of 1–2 years, whereas humans have an estimated generation time of 29 years (Langergraber et al., 2012, p. 15,717). Scaling the evolutionary persistence of ground squirrel snake recognition to this much longer human reproductive rate yields the potential for humans to retain adaptive information under relaxed selection extending back in time to the Pliocene age of Au. afarensis.
Sex Difference in Arboreal-Related Behavior of Young Children
The young of several mammalian species exhibit adult-like precocious behavior useful later in development that can be endangering, such as ground squirrel pups confronting a snake predator the first day they use vision for navigation (Coss, 1991a) or conspicuous stotting by Thomson’s gazelle fawns (Eudorcas thomsonii) when flushed by predators (Caro, 1986; Fitzgibbon, 1990). This expression of precocious adult-like behavior indicates that some essential neural circuits shaped by selection via adaptive behavior are installed on initial dendritic outgrowth that resists neural-circuit remodeling with later experience (e.g., Volkmar & Greenough, 1972). Higher-order dendritic branches that are labile to experience are not fully developed at this stage of brain development (see “Discussion”). Such early neural-circuit installation on stable portions of dendritic trees insures reliable behavioral expression needed later in development. Older patterns of neural circuitry installed early in development might also reveal the behavior of adult ancestors (Coss, 1991b), thus providing the rationale for studying behavioral precocity in young children.
The theoretical proposition for sexual dinichism in Au. afarensis by Susman et al. (1984) can be viewed as a core hypothesis useful for testing auxiliary hypotheses of Pliocene-age behavioral relicts in children. A philosophy of science argument for testing multiple auxiliary hypotheses using different methods that provide a “protective belt” for a core hypothesis is presented in Lakatos (1970, p. 191) and Meehl (1990, p. 110). This was the heuristical approach taken for a series of studies by Coss and Goldthwaite (1995) and Coss and Moore (2002). With this theoretical context in mind, I predicted that niche partitioning due to sexual-size dimorphism during hominin evolution would be reflected in children’s climbing and refuge-seeking behavior. For example, sex difference in climbing motivation was reported for a small sample of New Zealand kindergarten children (Halliday & McNaughton, 1982). These researchers mention that the most-preferred activity of girls was climbing playground structures while boys engaged in more high-intensity activity on the ground.
A more explicit follow-up question (Coss and Goldthwaite, 1995) was directed at determining whether girls climbed playground structures in American elementary schools more often than boys, a behavioral property that would characterize a desire to climb off the ground during recess. Sampling of 13 elementary schools based on age revealed reliably larger frequencies of girls 5 to 11 years old climbing on playground structures (except rings and swings) than same-age boys. Sex difference in climbing competence was then derived from the 1985–1989 US Product Safety Commission database, showing that girls less than 7 years of age were less likely to require hospitalization from falling from jungle gyms and monkey bars than same-aged boys. A sex difference was also apparent when young girls balanced with extended arms on a balance beam compared with boys who tended to balance less effectively with their arms by their sides (see Coss and Goldthwaite 1995, p. 128).
In a related study of refuge-site choice that included simulated climbing and hiding, Coss and Moore (2002) presented 4- to 6-year-old Israeli children with computer-generated images of a virtual tour of an African rock outcrop composed of a cluster of large boulders and an adjacent acacia tree. This scenario was not unlike the aforementioned scenario of refuge-site choice of Au. afarensis males and females for protection against predators at night. Girls were predicted to climb the acacia tree after seeing an image of a large male lion, whereas boys were predicted to seek shelter in a crevice between two boulders. After viewing the lion randomly from each of three refuge vantage points (the interior of the crevice, top of boulder, and acacia-tree canopy), the children were then requested to point to where they would go to feel safe. A reliably larger frequency of girls chose the acacia tree canopy for refuge than the other two sites, while a larger frequency of boys chose the crevice, but this locational preference in boys was not statistically significant. In another experiment in the same series, Coss and Moore (2002) requested 3- to 4-year-old American children without tree-climbing experience to climb silhouettes of trees with their fingers after being told that a lion was nearby. In this scenario, the girls differed reliably from the boys by selecting refuge sites closer to the crown edges of trees with wide crowns not unlike the aforementioned nighttime arboreal refuge selected by Old World monkeys (Ramakrishnan & Coss, 2000, 2001) or after detecting leopards (Busse, 1980). Follow-up game-like research addressing a similar question presented realistic models of a leopard or female mule deer (Odocoileus hemionus) suddenly from a 15-m distance to individual preschool children in a large preschool playground with a variety of climbable and concealing structures (Coss and Penkunas, 2016). Each child was prompted to go where she/he felt safe with the prediction that after seeing the leopard, the girls would differ from the boys by not seeking refuge in enclosed playground structures that afforded concealment. This prediction was supported because a reliably larger frequency of boys chose concealing structures to hide whereas the girls chose open areas where they could monitor the leopard. There was no sex difference in concealed or open refuge choice for the mule-deer model.
Children’s Nighttime Fear
Studies of children’s nighttime fear have had a long history in psychology. Hall (1897, p. 153) collected narratives of children’s fears, showing that girls were more fearful of “darkness” than boys, including the presence of imaginary ghosts. Developmental research on children’s nighttime fear has focused mostly on its clinical implications to childhood anxiety and experiential factors engendering fear and coping behavior (cf. Mooney et al., 1985; Zisenwine et al., 2013). Other studies focus on defining the properties of fearfulness and the role of imaginary creatures. For example, Bauer 1976, p. 70) interviewed nineteen 4- to 6-year-olds with the questions: “Are you afraid when you go to bed at night? What are your afraid of?” Audio recordings of children’s comments were then rated for categorizing content. Nighttime fear was reported by 52.6% of children, whereas monsters and ghosts were reported by 73.7% of these children, an incongruity suggesting that direct questioning about nighttime fear might engender inhibition in answering positively. Muris and colleagues (2001, p. 19) expanded this research on nighttime fear with a larger sample of 4- to 6-year-olds, reporting that 58.8% of children were fearful at night and that “imaginary creatures” were described by many more children than “animals.” Older 8- to 16-year-old children and adolescents were asked similar questions in a follow-up study about being fearful at night (Gordon et al., 2007). Reliable sex differences were found for all participants, with 72% of females and 54.6% of males reporting nighttime fear. A reliably larger frequency of females (21.9%) reported being fearful of the category “environmental threats” than males (14.5%). However, in these older children, imaginary creatures were reported by males and females at low frequencies of 5.4% and 5.2%, respectively. Together, these studies examining sex differences in arboreal tendencies and refuge choice in young children coupled with research on children’s nighttime fears set the stage for examining whether preschool children reveal an evolutionary persistence in assessing the location of a nighttime threat from either above, side, or below locations.
Experimental Questions and Hypotheses
The current experiment evaluates the hypothesis that human males and females would differ in assessing the location of a nighttime threat from either above, side, or below locations. This is one of the auxiliary hypotheses derived from the fundamental core hypothesis of sexual dinichism (i.e., niche partitioning) in the hominin lineage (Coss and Charles, 2004, p. 207) that was complemented by the aforementioned research on sex differences in children’s climbing motivation and refuge-site choice in an antipredator context (Coss and Goldthwaite, 1995; Coss and Moore, 2002; Coss and Penkunas, 2016). For the female component of the auxiliary hypothesis in which preschool girls and women were given choices of above, side, and below locations, it was predicted that the historically innate dread of something dangerous below them at night would predominate in both age classes. For the male component of the auxiliary hypothesis in which preschool boys and men were given the same three locations to choose, the historically innate dread of something dangerous approaching from their sides would predominate in both age classes. Neither sex was predicted to select the above location.
Materials and Methods
Participants
Two hundred nine typically developing 3- and 4-year-old children were sampled from 13 preschool and daycare settings in Sacramento, Solano, and Yolo counties, northern California. The group of boys consisted of 50 (3-year-olds) and 59 (4-year-olds). The group of girls consisted of 49 (3-year-olds) and 51 (4-year-olds). Only a subset of these children who reported fearfulness of something scary nearby at night was interviewed further about their locational fear. The ethnicities of these children were European (89%), South Asian (5.6%), East Asian (2%), Hispanic/Native American (2%), and African American (1.4%).
Adult participants consisted of 156 men and 336 women with an average age of 21 years, ranging in age from 19 to 36 years. The ethnicities of these adults were 48% European, 32% East Asian, and 20% South Asian, Hispanic/Native American, and African American. Only those adults who recalled being fearful at night as children continued to answer questions about their locational fears. All adult participants were enrolled in 6 upper division classes within the Psychology Department of the University of California, Davis. Sampling of child and adult participants was conducted over a 2-year period under Human Subjects protocols HSRC 91-243 and 93-321R from the University of California, Davis. The parents of all child participants were provided with consent forms describing the experiment that were signed and returned to the preschool and daycare settings. Adults were provided with a similar experiment-consent form as part of their survey instrument.
Survey Instrument and Procedure
To begin the survey, a child with parental permission was selected randomly for an interview in a separate room apart from the other children. Interviews were conducted by 12 trained female undergraduate students enrolled in consecutive upper division research courses emphasizing psychobiological experiments. Prior to development of a specific interview protocol, exploratory interviews of children discovered that preschool children were typically reluctant to answer a direct question of whether they were fearful of something scary at night. This reluctance was circumvented by using past-tense phrasing of “When you were little…”.
Each child was asked the following get-acquainted questions related to their sleeping situation followed by specific questions about their nighttime fear: (1) Record whether she/he sleeps alone or with another child or adult. (2) Record whether they sleep low or high off the ground (recorded as the on a mat on the floor, single bed, or bunk bed). (3) “Do you have a night light in your bedroom?” (4) If the child says “yes,” ask the following: “Do you like your night light on at night?” If the child says “no” to having a night light, ask the following: “Would you like a night light in your bedroom at night?” Tell the child: “Some kids like night lights because they think that scary things are in their rooms at night.” (5) Ask the child the following: “When you were little, did you ever think there were scary things in your room?” (Prompt only if there is language difficulty and record the child’s response as yes or no). (6) If the child responds with “yes,” proceed with the following statements. “Let’s pretend that you are little and this room (in here) is your bedroom. Can you tell me where you thought the scary thing might be?” The choices were as follows: (Above) near or above ceiling, as on the second floor; (Side) behind closet door/s, cabinets, or outside window/s; (Below) underneath the bed, on floor (or first floor). If the child selects more than one location, such as side and below, encourage the child to select just one location (i.e., the scariest or most likely place the scary thing might be). Record the child’s location choice and write down any unprompted comments about the scary thing.
The adult questionnaire distributed to students in each classroom described the questionnaire as an environmental-psychology survey requiring consent to participate. Questions on topics unrelated to the current study were presented first, followed by questions derived from the children’s in-person survey of nighttime fear: (1) When you were little, did you ever think there were scary things in your room at night? If the participant marked yes on the questionnaire, she/he was requested to continue answering only one of the three remaining questions. (2) Was the scary thing located above you, such as near or above ceiling, as on the second floor? (3) Was the scary thing located to your side, such as behind closet doors/s, cabinets, or outside windows? 4) Was the scary thing located below you, such as underneath the bed, on floor (or first floor)? The three location questions were presented in different orders in the distributed questionnaires.
Results
Qualitative Analyses
Frequencies of nighttime fear of scary things were almost identical for the 3-year-old boys and girls and very similar for the 4-year-old boys and girls. The following frequencies represent all children who were sampled, were fearful of something scary in their rooms, and selected one location where they thought the scary thing was located. For the 3 year-old boys (n = 50), 35 (70.00%) were fearful, with 32 children selecting only one location. For the 3 year-old girls (n = 49), 34 (69.39%) were fearful, with 33 selecting only one location. For the 4 year-old boys (n = 59), 44 (74.58%) were fearful, with 42 selecting only one location. For the 4 year-old girls (n = 51), 39 (76.47%) were fearful, with 33 selecting only one location.
A slightly larger proportion of adults remembered being fearful of something scary in their bedrooms at night. For the men (n = 156), 126 (80.77%) were fearful, with 124 selecting only one location. For the women (n = 336), 283 (84.23%) were fearful, with 280 selecting only one location.
Quantitative Analyses
The numbers of children and adults indicating only one location where they thought the scary thing was located in their bedrooms at night were examined in a contingency table. Comparisons of frequencies were made using multinomial log linear analyses with maximum likelihood estimations. Data partitioning (see Agresti, 2002 p. 82) was used to examine planned contrasts relevant to the threat-location hypotheses. Data from 3- and 4-year-old children were pooled because the age by location interactions for each sex were not statistically significant (boys: p = 0.186; girls: p = 0.448). For the entire contingency table of children and adults (Table 1), log-linear analysis revealed that the age by sex by location interaction was not statistically significant (Likelihood ratio χ22 (N = 544) = 1.604, p = 0.449), whereas the sex by location interaction was significant (Likelihood ratio χ22 (N = 544) = 29.847, p < 0.001) with a medium effect size (Cohen’s d = 0.50). As expected, the age by location interaction was not statistically significant (Likelihood ratio χ22 (N = 544) = 0.915, p > 0.5). Important for addressing the children’s hypothesis, planned contrast of sex by location for the 3- and 4-year-old children (Fig. 2a) revealed a statistically significant interaction (Likelihood ratio χ22 (n = 140) = 15.491, p < 0.001) with a medium effect size (Cohen’s d = 0.70). The interaction of sex by location for the adults (Fig. 2b) was also significant (Likelihood ratio χ22 (n = 404) = 16.337, p < 0.001), exhibiting a medium effect size (Cohen’s d = 0.41).
Frequency distributions of 3- to 4-year-old children (a) and adults (b) selecting locations for a scary thing near their beds at night. The interactions of sex by location were statistically significant for both age classes (p < 0.001). Planned contrasts showed that the side and below locations differed significantly for boys (p < 0.001, d = 1.38) and men (p < 0.001, d = 0.82), but not for girls and women
Comparison of age for each sex revealed that the preschool boys and men did not differ appreciably in the interaction of age by location (n = 198, p = 0.37). However, the preschool girls and women exhibited an interaction of age by location that was significantly similar (Likelihood ratio χ22 (n = 346) = 0.086, p > 0.95). Data partitioning further revealed that the preschool boys exhibited a significantly different frequency distribution for the three locations (χ22 (n = 74) = 46.730, p < 0.001) and specifically for the planned contrast of side (70.27%) and below (20.27%) locations (χ21 (n = 67) = 21.624, p < 0.001) that showed a large effect size (Cohen’s d = 1.38). This reliably larger proportion of boys selecting the side location for the scary thing supports the hypothetical construct of an evolutionary persistence of a cognitive relict derived from ancestral males anticipating a threat from their sides. The preschool girls also exhibited a significantly different frequency distribution for the three locations (χ22 (n = 74) = 25.844, p < 0.001), but their frequencies for side (40.91%) and below (51.52%) locations were not reliably different (n = 61, p = 0.37). This result for preschool girls does not support the hypothesis for a cognitive relict derived from ancestral females that distinguishes side and below locations for a potential threat at night.
Similar to the preschool boys, the men exhibited frequencies for the three locations that differed significantly (χ22 (n = 124) = 64.962, p < 0.001) and specifically for the planned contrast of side (63.71%) and below (29.03%) locations (χ21 (n = 115) = 16.476, p < 0.001) that showed a large effect size (Cohen’s d = 0.82). This finding supports the hypothesis that a larger proportion of men would select the side location compared with the below location. The frequency distribution of women for the three locations was also significant (χ22 (n = 280) = 108.392, p < 0.001). However, a planned contrast of side (42.86%) and below (49.64%) locations indicated that these frequencies did not differ appreciably (n = 259, p = 0.238), thereby disconfirming the hypothesis that a much larger proportion of women would select the below location.
Content Analyses of Described Scary Things
Since there were no predictive hypotheses associated with the children’s comments, the proceeding content analyses reveal descriptive findings with reliability estimates possible useful for subsequent research. Preschool girls were more vocal than the boys, with 42 girls (63.6%) naming scary things, such as dinosaurs/dragon, ghosts/demons, insects, snakes, spiders, monsters, and witches (i.e., imaginary threatening agents), compared with 34 boys (45.9%). Monsters were the predominant scary thing mentioned, with similar frequencies for boys (40.6%) and girls (35.7%). Cultural images of Halloween (ghosts and witches) were also expressed at similar frequencies by boys (20.6%) and girls (26.2%). Among the 13 girls who mentioned animals, 3 girls said cats and 4 said tigers. Only 1 boy mentioned a tiger among the animals 11 boys described. With cats and tigers grouped as felid agents, the interaction of sex by agent type yielded a significant interaction (χ21 (N = 24) = 5.906, p = 0.015).
A larger proportion of girls (n = 17) described threatening action by these agents than the boys (n = 9) associated with their choices of locations. Most of the girls’ action comments involved the below location: “coming up or come up” (below; n = 4), “tigers jumped up” (below), “pull me under bed” (below), “came out from under bed” (below), “crawling creatures” (below), “you can’t catch me” (below), “toys bumped me up and down” (below), “pinch-bite me” (below), “wiggling bed” (below), “will eat me” (below), “under bed they don’t like lights” (below), “biting hats behind closet doors” (side), and “in close to get me” (side). The action comments of the boys were similar, but emphasized the side location: “sits on head” (above), “ready to jump” (side), “sitting on cabinet” (side), “come out” (side), “hide in corners” (side), “came in from outside” (side), “come through door” (side), “looks under covers” (side), and “crawl under bed” (below). The interaction of sex by these locations was significant (χ22 (N = 26) = 16.526, p < 0.001) with the largest proportion of girls (88.2%) indicating the below location. It is important to note that none of the boys expressed action terms like “coming up, come up or jumped up” reflecting a threat from below.
Exploratory Study of Children Under 3 Years of Age
While the current experiment interviewed 3- to 4-year-old children for hypothesis testing, my research team also interviewed 18 children with parental permission younger than 3 years of age that provided insight into the earliest expression of nighttime fear with locational components. Thirteen children (8 girls, mean age = 30 months, range = 23–34 months; 5 boys, mean age = 28 months, range = 24–33 months) provided locational information on the scary thing vocally or by pointing. All 5 boys selected the side location whereas for the girls, one (12.5%) selected above, one (12.5%) selected the side, and six (75%) selected the below location. Although tentative in statistical inference due to the small sample size, this frequency distribution yielded a reliable sex by location interaction (χ22 (N = 13) = 13.380, p = 0.001). The youngest child, a 23 month-old girl, selected the below location with the comment “grab me,” an expression of threat assessment that might inspire further study of the nighttime fear of children younger than 3 years of age.
Discussion
The aim of the current experiment was to examine whether the nighttime fear of preschool children included historical expectations of the directions of where a “scary thing” was located relative to their beds. Preschool children were studied, in part, to corroborate adult recollections of their nighttime fears and the locations of scary things for subsequent study. For both age classes, sex differences in the choices of above, side, and below locations were evaluated using interviews of preschool children and adult questionnaires. Preschool children and adults were not expected to differ appreciably in their choices of the three locations. Indeed, the preschool boys and men exhibited similar frequency distributions of location choices while the distributions of the three locations selected by preschool girls and women were significantly similar.
Specific hypotheses of sex differences in assessment of the location of a scary thing were predominantly based on how the body size of extant great apes constrains arboreal nest construction as antipredator refuge and fossil evidence of sexual-size dimorphism in Australopithecus afarensis. Based on this fossil evidence, heavier-bodied males, unlike their female and juvenile counterparts, would likely have had limited access to safe places high in trees for constructing nests to mitigate nighttime predation. Relegated to nesting lower in trees but mostly on the ground, Au. afarensis males would have experienced a long history of natural selection operating on successful anticipation of ground-level threats and execution of evasive behavior. Thus, the explicit male component of the auxiliary hypothesis, derived from the core hypothesis of ancestral sexual dinichism affecting modern behavior (Coss and Charles, 2004), predicted that preschool boys and men would choose the side location for the scary thing. Consistent with this hypothesis, reliably larger proportions of preschool boys and men selected the side location compared with the below location, properties that contributed markedly to the statistically significant interaction of sex by the three locations for each age class (see Fig. 2).
For developing the female component of the auxiliary hypothesis, it was reasonable to proposed that lighter-bodied Au. afarensis females nesting high in trees at night would have anticipated potential danger from large-bodied felids on the ground or coming up to attack. Therefore, females in both age classes were predicted to select the below location relative to their beds. Although larger frequencies of preschool girls and women selected the below location more than the side location for the scary thing, the planned contrasts of side and below locations were not statistically significant for either age class, thereby disconfirming this feature of the female hypothesis. As predicted, both age classes selected the above location at low frequencies.
While the reliable statistical interactions of sex by location in both age classes support the fundamental property of the auxiliary hypothesis driven predominantly by the low frequencies of males selecting the below location, the relatively similar frequencies of females selecting side and below locations require further interpretation. In retrospect, this disconfirmation of my prediction that side and below locations would be distinguished by females reflects, in part, my theoretical concentration on the last representative of Au. afarensis ~ 3 Ma as the beginning of relaxed selection on female arboreality rather than considering the evolutionary effects of increased ground nesting by females. That is, the gradual diminution of female arboreality during the likely transformation period of Australopithecus afarensis to Homo (see Villmoare et al., 2015) would have been accompanied by positive natural selection on ground-nesting females for enhanced anticipation of predatory threats from their sides. This period of derived generic transformation coincides roughly with the decline in lake levels after 2.7 Ma (Trauth et al., 2007), a decrease in humid forests, and expansion of grassland-savanna habitats (cf. Bobe & Behrensmeyer, 2004; Kingston & Harrison, 2007) that would have also engendered natural selection for greater biomechanical efficiency in walking (Carey & Crompton, 2005). Irrespective of relaxed selection on assessment of predatory threats from below, an additional 2 Ma for positive selection operating on female anticipation of ground-level threats at night would encompass the advent of Homo erectus, archaic humans, and anatomically modern humans. This total 2.7-Ma time frame of natural selection might be sufficient to explain the large proportion of females choosing of the side location in the current experiment.
The comments of the preschool children examined by content analyses characterized how the children evaluated their nighttime threats. A larger proportion of girls made unprompted comments than the boys did; albeit, their descriptions of monsters, ghosts, and dangerous animals were similar. However, their increased spontaneity in making comments might reflect developmental differences in the rate of language proficiency at the ages tested (Lange et al., 2016; Wallentin, 2009). A putative sex difference in vocabulary might account for more girls making comments relative to the boys, but it would not explain why girls provided reliably more comments of dangerous actions by the scary thing coming from below their beds.
The development stability of nighttime fear at 3- and 4-years of age was expected, in part, due to the past-tense phrasing of the question of “When you were little…” The preschool boys and girls from both age classes were similarly fearful of the scary thing at night, with an average frequency of fearfulness (72.73%) closely approximating the frequency (72.9%) reported for older females by Gordon and colleagues (2007); although unlike the current study, the juvenile and adolescent males in their study were reliably less fearful (54.6%) than the females. Moreover, in the current study, 83.13% of adults reported experiencing nighttime fear as children that suggests a robust continuity of salient recollections.
In further study of the adult remembrances of nighttime fears as children, Cathleen Hunt, one of the undergraduate interviewers of preschool children, continued to pursue research at the graduate level, examining cross-national differences by Americans, Batswana, Danes, Germans, Hondurans, and Zimbabweans. Hunt (1997) reported a significant main effect of sex, in which females indicated that they were more fearful than males of a scary presence under their beds as children. The statistical interaction of sex and country was also significant, in which American, Batswana, and Danish women were more fearful of something scary below their beds than males, whereas Honduran and Zimbabwean men and women did not differ appreciably. This latter result suggests that cultural factors during child development might restructure the locational aspects of children’s nighttime fear.
The precocious expression of nighttime fear in children under 3 years of age that included the locational assessment of danger is relevant to my argument about the developmental stability of evolved neural circuits installed early in the developing brain for adaptive utility later in life. Based on this exploratory evidence, it is reasonable to argue that the installation of interneural connections mediating the locational attributes of nighttime fear occurs on the initial outgrowth of dendritic branches of neocortical neurons in prefrontal (Mrzljak et al., 1992) and parietal cortices during the first year of life. This is the time frame of extensive postnatal gene expression for neurotrophins shaping neuronal morphology (Wong et al., 2009) and the overproduction of synapses on dendritic spines that are pruned later in development (Huttenlocher & Dabholkar, 1997; Petanjek et al., 2011). Such synaptic tailoring requires appropriate experience for stabilizing dendritic spines for current and later utility (Chen & Zuo, 2014; Basu & Lamprecht, 2018). Despite broad neuronal reorganization with synaptic pruning coupled with “experience-dependent” growth of higher-order dendrites (cf. Black & Greenough, 1986; Petit et al., 1988; Volkmar & Greenough, 1972), the synaptic connections installed on initial dendritic outgrowth subserving survival-oriented behaviors must be stabilized already without explicit predator-related experiences in order to await the stochastic circumstances of predator encounters later in life. As an example of an awaiting antipredator system organized on the initial outgrowth of dendritic branches (Coss, 1991a, 1991b), California ground squirrels reared in isolation until adulthood still express innate expectations that snakes can lurk in dark burrow entrances (Tromborg & Coss, 2015). Similar isolation rearing of laboratory rats is known to suppress the growth of higher-order dendrites in laboratory rats (Volkmar & Greenough, 1972).
Coincidental with dendritic spine overproduction and pruning in humans, the growth rate of neocortical grey matter is linear during the first year, stabilizing in growth rate after 2 years of age (Gilmore, Knickmeyer & Gao, 2018). Specifically relevant to the onset of assessing imaginary locational threats, this 1- to 2-year time frame is accompanied by the increasing thickness of neocortical layers 2 and 3, that contributes to surface-area expansion and cortical folding of temporal, prefrontal, and parietal regions compared with other neocortical brain regions (Landing et al., 2002; Li et al., 2013, 2014). In particular, the posterior parietal cortex of adults employs spatial coordinates for modulating attention and visual imagery (Corbetta et al., 2000; Ishai et al., 2000) relevant to the locational properties of nighttime fear in children.
The locations of imaginary images engendering nighttime fear of young children are clearly contextual with respect to bedroom geometry and being alone in the dark (see El Rafihi-Ferreira et al., 2019). The emergence of scary imaginary images in the dark and their directional components in young children could reflect neural-circuit exercising as contextual rehearsals for later use in a world that exists today only in developing countries with dangerous felid predators that attack at night (see Corbett, 1948; Packer et al., 2011, 2019). With evidence that adult remembrances are relatively accurate representations of childhood nighttime fear, subsequent research might consider examining the role of social facilitation (see Rachman, 1977) in shaping children’s nighttime fear and related fears that are also expressed in adulthood.
Data Availability and Code Availability
All the data for statistical analyses are presented in this manuscript.
Change history
01 April 2020
In the last paragraph under "Discussion" section, the sentence "The emergence of scary imaginary images in the dark and their directional components in young children could reflect neural-circuit exercising as contextual rehearsals for later use in a world that exists today that attack at night (see Corbett, 1948; Packer et al., 2011, 2019)." should read as "The emergence of scary imaginary images in the dark and their directional components in young children could reflect neural-circuit exercising as contextual rehearsals for later use in a world that exists today only in developing countries with dangerous felid predators that attack at night (see Corbett, 1948; Packer et al., 2011, 2019)."
References
Agresti, A. (2002). Categorical data analysis (2nd ed.). Wiley
Alanen, J., Levola, J. V., & Kvist, M. (2001). Ankle joint complex mobility of children 7 to 14 years old. Journal of Pediatric Orthopaedics, 21(6), 731–737. https://doi.org/10.1097/01241398-200111000-00006
Alemseged, Z., Spoor, F., Kimbel, W. H., Bobe, R., Geraads, D., Reed, D., & Wynn, J. G. (2006). A juvenile early hominin skeleton from Dikika Ethiopia. Nature, 443(7109), 296–301
Anderson, J. R. (1998). Sleep, sleeping sites, and sleep-related activities: Awakening to their significance. American Journal of Primatology, 46(1), 63–75. https://doi.org/10.1002/(SICI)1098-2345(1998)46:1%3c63::AID-AJP5%3e3.0.CO;2-T
Arenz, C. L., & Leger, D. W. (1997). The antipredator vigilance of adult and juvenile thirteen-lined ground squirrels (Sciuridae: Spermophilus tridecemlineatus): Visual obstruction and simulated hawk attacks. Ethology, 103(11), 945–953. https://doi.org/10.1111/j.1439-0310.1997.tb00136.x
Bailey, G. N., & King, G. C. P. (2010). Landscapes of human evolution: model and methods of tectonic geomorphology and the reconstruction of hominin landscapes. Journal of Human Evolution, 60(3), 257–280. https://doi.org/10.1016/j.jhevol.2010.01.004
Baldwin, D. V. (2013). Primitive mechanisms of trauma response: An evolutionary perspective on trauma-related disorders. Neuroscience and Biobehavioral Reviews, 37(8), 1549–1566. https://doi.org/10.1016/j.neubiorev.2013.06.004
Barrett, H. C. (2005). Adaptations to predators and prey. In D. M. Buss (Ed.), Handbook of evolutionary psychology (pp. 200–233). Wiley
Barry, J. C. (1987). Large carnivores (Canidae, Hyaenidae, Felidae) from Laetoli. In M. D. Leakey & J. M. Harris (Eds.), Laetoli: A Pliocene site in Tanzania (pp. 235–258). Clarendon Press
Basu, S., & Lamprecht, R. (2018). The role of actin cytoskeleton in dendritic spines in the maintenance of long-term memory. Frontiers in Molecular Neuroscience, 11(143), 1–9. https://doi.org/10.3389/fnmol.2018.00143
Bauer, D. H. (1976). An exploratory study of developmental changes in children’s fears. The Journal of Child Psychology and Psychiatry, 17(1), 69–74. https://doi.org/10.1111/j.1469-7610.1976.tb00375.x
Beighton, P., Soloman, L., & Soskolne, C. L. (1973). Articular mobility in an African population. Annals of the Rheumatic Diseases, 32(5), 413–418. https://doi.org/10.1136/ard.32.5.413
Black, J. E., & Greenough, W. T. (1986). Induction of pattern in neural structure by experience: Implications for cognitive development. In M. E. Lamb, A. L. Brown, & B. Rogoff (Eds.), Advances in developmental psychology, 4 (pp. 1–50). Erlbaum
Boaz, N. T., Howell, F. C., & McCrossin, M. L. (1982). Faunal age of the Usno, Shungura B and Hadar Formations, Ethiopia. Nature, 300, 633–635. https://doi.org/10.1038/300633a0
Bobe, R., & Behrensmeyer, A. K. (2004). The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeography, Palaeoclimatology, Palaeoecology, 207(3–4), 399–420. https://doi.org/10.1016/j.palaeo.2003.09.033
Boesch, C. (1991). The effects of leopard predation on grouping patterns in forest chimpanzees. Behaviour, 117(3/4), 220–242. https://www.jstor.org/stable/4534940
Bowlby, J. (1969/1982). Attachment and loss. Vol. 1. Attachment. New York: Basic Books
Brain, C. K. (1970). New finds at the Swartkrans australopithecine site. Nature, 225, 1112–1119. https://doi.org/10.1038/2251112a0
Brain, C. K. (1981). The hunters or the hunted? University of Chicago Press
Busse, C. (1980). Leopard and lion predation upon chacma baboons living in the Moremi Wildlife Reserve. Botswana Notes & Records, 12, 15–21. http://www.jstor.com/stable/40980790
Butler, M. A., Schoener, T. W., & Losos, J. B. (2000). The relationship between sexual size dimorphism and habitat use in Greater Antillean Anolis lizards. Evolution, 54(1), 259–272. https://doi.org/10.1111/j.0014-3820.2000.tb00026.x
Cameron, D. W. (2004). Faciodental sexual dimorphism and species variability in the Hungarian dryopithecine. Palaeontographia Italica, 90, 83–96.
Caro, T. M. (1986). The functions of stotting in Thomson’s gazelles: Some tests of the predictions. Animal Behaviour, 34(3), 663–684. https://doi.org/10.1016/S0003-3472(86)80052-5
Carey, T. S., & Crompton, R. H. (2005). The metabolic costs of ‘bent-hip, bent-knee’ walking in humans. Journal of Human Evolution, 48(1), 25–44. https://doi.org/10.1016/j.jhevol.2004.10.001
Chemero, A. (2003). An outline of a theory of affordances. Ecological Psychology, 15(2), 181–195. https://doi.org/10.1207/S15326969ECO1502_5
Chen, CC., Lu, J., & Zuo, Y. (2014). Spatiotemporal dynamics of dendritic spines in the living brain. Frontiers in Neuroanatomy, 8(28), 1–7. https://doi.org/10.3389/fnana.2014.00028
Christiansen, P. (2013). Phylogeny of the sabertoothed felids (Carnivora: Felidae: Machairodontinae). Cladistics, 29(5), 543–559. https://doi.org/10.1111/cla.12008
Corbett, J. E. (1948). The man-eating leopard of Rudraprayag. Oxford University Press
Corbetta, M., Kincade, J. M., Ollinger, J. M., McAvoy, M. P., & Shulman, G. L. (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3, 292–297. https://doi.org/10.1038/73009
Coss, R. G. (1991a). Context and animal behavior III: The relationship between early development and evolutionary persistence of ground squirrel antisnake behavior. Ecological Psychology, 3(4), 277–315. https://doi.org/10.1207/s15326969eco0304_1
Coss, R. G. (1991b). Evolutionary persistence of memory-like processes. Concepts in Neuroscience, 2(2), 129–168
Coss, R. G. (1993). Evolutionary persistence of ground squirrel antisnake behavior: Reflections on Burton’s commentary. Ecological Psychology, 5(2), 171–194. https://doi.org/10.1207/s15326969eco0502_4
Coss, R. G. (1999). Effects of relaxed natural selection on the evolution of behavior. In S. A. Foster & J. A. Endler (Eds.), Geographic variation in behavior: Perspectives on evolutionary mechanisms (pp. 180–208). Oxford University Press
Coss, R. G., & Charles, E. P. (2004). The role of evolutionary hypotheses in psychological research: Instincts, affordances, and relic sex differences. Ecological Psychology, 16(3), 199–236. https://doi.org/10.1207/s15326969eco1603_3
Coss, R. G., & Goldthwaite, R. O. (1995). The persistence of old designs for perception. In N. S. Thompson (Ed.), Perspectives in ethology 11: Behavioral design (pp. 83–148). Plenum Press
Coss, R. G., & Moore, M. (2002). Precocious knowledge of trees as antipredator refuge in preschool children: An examination of aesthetics, attributive judgments and relic sexual dinichism. Ecological Psychology, 14(4), 181–222. https://doi.org/10.1207/S15326969ECO1404_1
Coss, R. G., & Penkunas, M. J. (2016). Sex difference in choice of concealed or exposed refuge sites by preschool children viewing a model leopard in a playground simulation of antipredator behavior. International Journal of Psychological Research, 9(2), 8–19. https://doi.org/10.21500/20112084.2325
Cowlishaw, G. (1997). Refuge use and predation risk in a desert baboon population. Animal Behaviour, 54(2), 241–253. https://doi.org/10.1006/anbe.1996.0466
Crompton, R. H. (2016). The hominins: a very conservative tribe? Last common ancestors, plasticity and ecomorphology in Hominidae. Or what’s in a name? Journal of Anatomy, 228(4), 686–699.https://doi.org/10.1111/joa.12424
Damiano, M. (2015). Using the morphology of the hominoid distal fibula to interpret arboreality in Australopithecus afarensis. Journal of Human Evolution, 85, 136–148. https://doi.org/10.1016/j.jhevol.2015.06.002
de Heinzelin, J., Clark, J. D., White, T., Hart, W., Renne, P., Woldegabriel, G., Beyene, Y., & Vrba, E. (1999). Environment and behavior of 2.5-million-year-old Bouri hominids. Science, 284(5414), 625–629. https://www.jstor.org/stable/2897932
deMenocal, P. (1995). Plio-Pleistocene African climate. Science, 270(5233), 53–59. http://www.jstor.com/stable/2888211
Drapeau, M. S. M., Ward, C. V., Kimbel, W. H., Johanson, D. C., & Rak, Y. (2005). Associated cranial and forelimb remains attributed to Australopithecus afarensis from Hadar Ethiopia. Journal of Human Evolution, 48(6), 593–642. https://doi.org/10.1016/j.jhevol.2005.02.005
El Rafihi-Ferreira, R., Lewis, K. M., McFayden, T., & Ollendick, T. H. (2019). Predictors of nighttime fears and sleep problems in young children. Journal of Child and Family Studies, 28, 941–949. https://doi.org/10.1007/s10826-019-01332-9
Fan, P. F., & Jiang, X. L. (2008). Sleeping sites, sleeping trees, and sleep-related behaviors of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. American Journal of Primatology, 70(2), 153–160. https://doi.org/10.1002/ajp.20470
Fitzgibbon, C. D. (1990). Anti-predator strategies of immature Thomson’s gazelles: Hiding and the prone response. Animal Behaviour, 40(5), 846–855. https://doi.org/10.1016/S0003-3472(05)80985-6
Garvin, H. M. (2012). The effects of living conditions on human cranial and postcranial sexual dimorphism. Ph.D. Dissertation, John Hopkins University. UMI 3537330.
Gilmore, J. H., Knickmeyer, R. C., & Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19, 123–137. https://doi.org/10.1038/nrn.2018.1
Goldthwaite, R. O., Coss, R. G., & Owings, D. H. (1990). Evolutionary dissipation of an antisnake system: Differential behavior by California and Arctic ground squirrels in above- and below-ground contexts. Behaviour, 112(3/4), 246–269. http://www.jstor.com/stable/4534840
Gordon, A. D., Green, D. J., & Richmond, B. G. (2008). Strong postcranial size dimorphism in Australopithecus afarensis: results from two new resampling methods for multivariate data sets with missing data. American Journal of Physical Anthropology, 135(3), 311–328. https://doi.org/10.1002/ajpa.20745
Gordon, J., King, N., Gullone, E., Muris, P., & Ollendick, T. H. (2007). Nighttime fears of children and adolescents: Frequency, content, severity, harm expectations, disclosure, and coping behaviours. Behaviour Research and Therapy, 45(10), 2464–2472. https://doi.org/10.1016/j.brat.2007.03.013
Grabowski, M., Hatala, K. G., Jungers, W. L., & Richmond, B. G. (2015). Body mass estimates of hominin fossils and the evolution of human body size. Journal of Human Evolution, 85, 75–93. https://doi.org/10.1016/j.jhevol.2015.05.005
Haile-Selassie, Y., Latimer, B. M., Alene, M., Deino, A. L., Gibert, L., Melillo, S. M., & Lovejoy, C. O. (2010). An early Australopithecus afarensis postcranium from Woranso-mille, Ethiopia. Proceedings of the National Academy of Sciences, 107(27), 12121-12126https://www.pnas.org/cgi/doi/10.1073/pnas.1004527107
Hall, G. S. (1897). A study of fears. The American Journal of Psychology, 9(2), 147–249. https://www.jstor.org/stable/1410940
Halliday, J., & McNaughton, S. (1982). Sex differences in play at kindergarten. New Zealand Journal of Education Studies, 17(2), 161–170
Hughes, A. (1977). The topography of vision in mammals of contrasting life style: Comparative optics and retinal organisation. In F. Cresutelli (Ed.), Handbook of Sensory Physiology, 7(5) (pp. 613–756). Springer
Hunt, C. B. (1997). A cross-cultural examination of daytime and nighttime fears as evolved adaptive mechanisms. Master’s Thesis, California State University, Sacramento, Sacramento, California
Huttenlocher, P. R., & Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387(2), 167–178. https://doi.org/10.1002/(SICI)1096-9861(19971020)387:2%3c167::AID-CNE1%3e3.0.CO;2-Z
Isbell, L. A., Bidner, L. R., Van Cleave, E. K., Matsumoto-Oda, A., & Crofoot, M. C. (2018). GPS-identified vulnerabilities of savannah-woodland primates to leopard predation and their implications for early hominins. Journal of Human Evolution, 118, 1–13. https://doi.org/10.1016/j.jhevol.2018.02.003
Ishai, A., Ungerleider, L. G., & Haxby, J. V. (2000). Distributed neural systems for the generation of visual images. Neuron, 28(3), 979–990. https://doi.org/10.1016/S0896-6273(00)00168-9
Jenny, D., & Zuberbühler, K. (2005). Hunting behavior in West African forest leopards. African Journal of Ecology, 43(3), 197–200. https://doi.org/10.1111/j.1365-2028.2005.00565.x
Kappelman, J., Ketcham, R. A., Pearce, S., Todd, L., Akins, W., Colbert, M. W., & Witzel, A. (2016). Perimortem fractures in Lucy suggest mortality from fall out of tall tree. Nature, 537, 503–507. https://doi.org/10.1038/nature19332
Kimbel, W. H., Johanson, D. C., & Rak, Y. (1994). The first skull and other new discoversies of Australopithecus afarensis at Hadar, Ethiopia. Nature, 368, 449–451. https://doi.org/10.1038/368449a0
King, G., & Bailey, G. (2006). Tectonics and human evolution. Antiquity, 80(308), 265–286. https://doi.org/10.1017/S0003598X00093613
Kingston, J. D., & Harrison, T. (2007). Isotopic dietary reconstructions of Pliocene herbivores at Laetoli: Implications for early hominin paleoecology. Palaeogeography, Palaeoclimatology, Palaeoecology, 243(3–4), 272–306. https://doi.org/10.1016/j.palaeo.2006.08.002
Koops, K., Humle, T., Sterck, E. H. M., & Matsuzawa, T. (2007). Ground-nesting by the chimpanzees of the Nimba Mountains, Guinea: environmentally or socially determined? American Journal of Primatology, 69(4), 407–419. https://doi.org/10.1002/ajp.20358
Koops, K., McGrew, W. C., de Vries, H., & Tetsuro, M. (2012). Nest-building by chimpanzees (Pan troglodytes verus) at Seringbara, Nimba mountains: Antipredation, thermoregulation, and antivector hypotheses. International Journal of Primatology, 33, 356–380. https://doi.org/10.1007/s10764-012-9585-4
Lakatos, I. (1970). Falsificationism and the methodology of scientific research programmes. In I. Lakatos & A. Musgrave (Eds.), Criticism and the growth of knowledge (pp. 91–196). Cambridge, England: Cambridge University Press. http://www.csun.edu/~vcsoc00i/classes/s680f11/Lakatos.pdf
Land, M. F. (2013). Animal vision: Rats watch the sky. Current Biology, 23(14), R612. https://doi.org/10.1016/j.cub.2013.06.015
Landing, B. H., Shankle, W. R., Hara, J., Brannock, J., & Fallon, J. H. (2002). The development of structure and function in the postnatal human cerebral cortex from birth to 72 months: Changes in the thickness of layers II and III co-relate to the onset of new age-specific behaviors. Pediatric Pathology & Molecular Medicine, 21(3), 321–342. https://doi.org/10.1080/pdp.21.3.321.342
Lange, B. P., Euler, H. A., & Zaretsky, E. (2016). Sex differences in language competence of 3- to 6-year-old children. Applied Psycholinguistics, 37(6), 1417–1438. https://doi.org/10.1017/S0142716415000624
Langergraber, K. E., Prüfer, K., Rowney, C., Boesch, C., Crockford, C., Fawcett, K., & & Vigilant, L. . (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proceedings of the National Academy of Sciences USA, 109(39), 15716–15721. https://doi.org/10.1073/pnas.1211740109
Latimer, B., Ohman, J. C., & Lovejoy, C. O. (1987). Talocrural joint in African hominoids: Implications for Australopithecus afarensis. American Journal of Physical Anthropology, 74(2), 155–175. https://doi.org/10.1002/ajpa.1330740204
Leigh, S. R., & Shea, B. T. (1995). Ontogeny and the evolution of adult body size dimorphism in apes. American Journal of Primatology, 36(1), 37–60. https://doi.org/10.1002/ajp.1350360104
Leonard, W. R., & Hegmon, M. (1987). Evolution of P3 morphology in Australopithecus afarensis. American Journal of Physical Anthropology, 73, 41–63. https://doi.org/10.1002/ajpa.1330730105
Leonard, W. R., & Robertson, M. L. (1994). Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. American Journal of Human Biology, 6(1), 77–88. https://doi.org/10.1002/ajhb.1310060111
Li, G., Nie, J., Wang, L., Shi, F., Lin, W., Gilmore, J. H., & Shen, D. (2013). Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cerebral cortex, 23(11), 2724-2733
Li, G., Wang, L., Shi, F., Lyall, A. E., Lin, W., Gilmore, J. H., & Shen, D. (2014). Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. The Journal of Neuroscience, 34(12), 4228–4238
Lovejoy, C. O., Johanson, D. C., & Coppens, Y. (1982). Hominind upper limb bones recovered from the Hadar Formation: 1974–1977 collections. American Journal of Physical Anthropology, 57(4), 637–649. https://doi.org/10.1002/ajpa.1330570409
Marler, P., & Evans, C. (1996). Bird calls: just emotional displays or something more? Ibis, 138(1), 26–33. https://doi.org/10.1111/j.1474-919X.1996.tb04310.x
Masao, F. T., Ichumbaki, E. B., Cherin, M., Barili, A., Boschian, G., Iurino, D. A., & Manzi, G. (2016). New footprints from Laetoli (Tanzania) provide evidence for marked body size variation in early hominins. eLife, 5, e19568. https://doi.org/10.7554/eLife.19568
McHenry, H. M. (1992). Body size and proportions in early hominids. American Journal of Physical Anthropology, 87(4), 407–431. https://doi.org/10.1002/ajpa.1330870404
McHenry, H. M., & Coffing, K. (2000). Australopithecus to Homo: Transformations in body and mind. Annual Review of Anthropology, 29, 125–146. https://doi.org/10.1146/annurev.anthro.29.1.125
Meehl, P. E. (1990). Appraising and amending theories: The strategy of Lakatosian defense and two principles that warrant it. Psychological Inquiry, 1(2), 108–141. https://doi.org/10.1207/s15327965pli0102_1
Melillo, S. M. (2016). The shoulder girdle of KSD-VP-1/1. In Y. Haile-Selassie & D. F. Su (Eds.), The postcranial anatomy of Australopithecus afarensis: New insights from KSD-VP-1/1, vertebrate paleobiology and paleoanthropology (pp. 113–141). Springer. https://doi.org/10.1007/978-94-017-7429-1_6
Miller, P. M., & Commons, M. L. (2010). The benefits of attachment parenting for infants and children: A behavioral developmental view. Behavioral Development Bulletin, 16(1), 1–14. https://doi.org/10.1037/h0100514
Mobbs, D., Haga, C. C., Dalgleish, T., Silston, B., & Prévost, C. (2015). The ecology of human fear: survival optimization and the nervous system. Frontiers in neuroscience, 9(55), 1–22. https://doi.org/10.3389/fnins.2015.00055
Mooney, K. C., Graziano, A. M., & Katz, J. N. (1985). A Factor analytic investigation of children’s nighttime fear and coping responses. The Journal of Genetic Psychology, 146(2), 205–215. https://doi.org/10.1080/00221325.1985.9914448
Mrzljak, L., Uylings, H. B. M., Kostovic, I., & van Eden, C. G. (1992). Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. The Journal of Comparative Neurology, 316, 485–496. https://doi.org/10.1002/cne.903160408
Muris, P., Merckelbach, H., Ollendick, T. H., King, N. J., & Bogie, N. (2001). Children’s nighttime fears: Parent-child ratings of frequency, content, origins, coping behaviors, and severity. Behaviour Research and Therapy, 39(1), 13–28. https://doi.org/10.1016/S0005-7967(99)00155-2
Ndou, R. (2018). The significance of the supratrochlear aperture (STA) in elbow range of motion: An anatomical study. Anatomical Science International, 93, 88–97. https://doi.org/10.1007/s12565-016-0376-4
Packer, C., Swanson, A., Ikanda, D., & Kushnir, H. (2011). Fear of darkness, the full moon and the nocturnal ecology of African lions. PLoS ONE, 7, e22285. https://doi.org/10.1371/journal.pone.0022285
Packer, C., Shivakumar, S., Athreye, V., Craft, M. E., Dhanwatey, H., Dhanwatery, P., & Fountain-Jones, N. M. (2019). Species-specific spatiotemporal patterns of leopard, lion and tiger attacks on humans. Journal of Applied Ecology, 56(3), 585. https://doi.org/10.1111/1365-2664.13311
Petanjek, Z., Judaš, M., Šimic, G., Rasin, M. R., Uylings, H. B. M., Rakic, P., & Kostović, I. (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences USA, 108, 13281–13286. https://doi.org/10.1073/pnas.1105108108
Petit, T. L., Leboutillier, J. C., Gregorio, A., & Libstug, H. (1988). The pattern of dendritic development in the cerebral cortex of the rat. Developmental Brain Research, 41(1–2), 209–219. https://doi.org/10.1016/0165-3806(88)90183-6
Pi, J. S., Veà, J. J., & Serrallonga, J. (1997). Did the first hominids build nests? Current Anthropology, 38(5), 914–916. https://www.jstor.org/stable/10.1086/204682
Plavcan, J. M. (1994). Comparison of four simple methods for estimating sexual dimorphism in fossils. American Journal of Physical Anthropology, 94(4), 465–476. https://doi.org/10.1002/ajpa.1330940403
Pontzer, H., Brown, M. H., Raichlen, D. A., Dunsworth, H., Hare, B., Walker, K., & Ross, S. R. (2016). Metabolic acceleration and the evolution of human brain size and life history. Nature, 533, 390–392. https://doi.org/10.1038/nature17654
Pruetz, J. D., Fulton, S. J., Marchant, L. F., McGrew, W. C., Schiel, M., & Waller, M. (2008). Arboreal nesting as anti-predator adaptation by savanna chimpanzees (Pan troglodytes verus) in southeastern Senegal. American Journal of Primatology, 70(4), 393–401. https://doi.org/10.1002/ajp.20508
Qvindesland, A., & Jónsson, H. (1999). Articular hypermobility in Icelandic 12-year-olds. Rheumatology, 38(10), 1014–1016. https://doi.org/10.1093/rheumatology/38.10.1014
Rachman, S. (1977). The conditioning theory of fear-acquisition: A critical examination. Behaviour Research and Therapy, 15(5), 375–387. https://doi.org/10.1016/0005-7967(77)90041-9
Ramakrishnan, U., & Coss, R. G. (2000). A comparison of the sleeping behavior of three sympatric primates: A preliminary report. Folia Primatologica, 72, 51–53.
Ramakrishnan, U., & Coss, R. G. (2001). Strategies used by Bonnet Macaques (Macaca radiata) to reduce predation risk while sleeping. Primates, 42, 193–206. https://doi.org/10.1007/BF02629636
Rehkämper, G., Anette Perrey, A., Werner, C. W., Opfermann-Rüngeler, C., & Görlach, A. (2000). Visual perception and stimulus orientation in cattle. Vision Research, 40, 2489–2497. https://doi.org/10.1016/S0042-6989(00)00113-9
Remis, M. J. (1995). Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. American Journal of Physical Anthropology, 97(4), 413–433. https://doi.org/10.1002/ajpa.1330970408
Reno, P. L., & Lovejoy, C. O. (2015). From Lucy to Kadanuumuu: balanced analyses of Australopithecus afarensus assemblages confirm only moderate skeletal dimorphism. PeerJ, 3, e925. https://doi.org/10.7717/peerj.925
Rogers, T. L. (1999). A visual method of determining the sex of skeletal remains using the distal humerus. Journal of Forensic Sciences, 44(1), 57–60. https://doi.org/10.1520/JFS14411J
Sacragit, A., & Ikeda, T. (1995). Sex identification from the distal fibula. International Journal of Osteoarchaeology, 5, 139–143. https://doi.org/10.1002/oa.1390050205
Schaller, G. B. (1963). The mountain gorilla. University of Chicago Press
Schoener, T. W. (1969). Models of optimal size for solitary predators. American Naturalist, 103(931), 277–313. http://www.jstor.com/stable/2459329
Scott, J. E., & Stroik, L. K. (2006). Bootstrap tests of significance and the case for humanlike skeletal-size dimorphism in Australopithecus afarensis. Journal of Human Evolution, 51(4), 422–428. https://doi.org/10.1016/j.jhevol.2006.06.001
Sirkin, D., Venolia, G., Tang, J., Robertson, G., Kim, T., Inkpen, K., & Sinclair, M. (2011). Motion and atttention in a kinetic videoconferencing proxy. In P. Campos, N. Graham, J. Jorge, N. Nunes, P. Palanque, & M. Winckler (Eds.) Human-computer interaction–Lecture notes in computer science, 6946 (pp. 162–180). Berlin: Springer. https://doi.org/10.1007/978-3-642-23774-4_16
Slatkin, M. (1984). Ecological causes of sexual dimorphism. Evolution, 622-630.https://www.jstor.org/stable/2408711
Soucie, J. M., Wang, C., Forsyth, A., Funk, S., Denny, M., Roach, K. E., & Boone, D. (2011). Range of motion measurements: Reference values and a database for comparison studies. Haemophilia, 17(3), 500–507. https://doi.org/10.1111/j.1365-2516.2010.02399.x
Spörrle, M., & Stich, J. (2010). Sleeping in safe places: An experimental investigation of human sleeping place preferences from an evolutionary perspective. Evolutionary Psychology, 8(3), 405–419. https://doi.org/10.1177/147470491000800308
Stern, J. T. Jr., & Susman, R. L. (1983). The locomotor anatomy of Australopithecus afarensis. American Journal of Physical Anthropology, 80(3), 279–317. https://doi.org/10.1002/ajpa.1330600302.
Stern, J. T. Jr. (2002). Climbing to the top: A personal memoir of Australopithecus afarensis. Evolutionary Anthropology, 9(3), 113–133. https://doi.org/10.1002/1520-6505(2000)9:3<113::AID-EVAN2>3.0.CO;2-W
Stewart, F. A., & Pruetz, J. D. (2013). Do chimpanzee nests serve an anti-predatory function? American Journal of Primatology, 75(6), 593–604. https://doi.org/10.1002/ajp.22138
Susman, R. L., Stern, J. T., Jr., & Jungers, W. L. (1984). Arboreality and bipedality in the Hadar hominids. Folia Primatologica, 43(2–3), 113–156. https://doi.org/10.1159/000156176
Tallman, S. D., & Blanton, A. I. (2020). Distal humerus morphological variation and sex estimation in modern Thai individuals. Journal of Forensic Sciences, 65(2), 361–371. https://doi.org/10.1111/1556-4029.14218
Trauth, M. H., Maslin, M. A., Deino, A. L., Strecker, M. R., Bergner, A. G. N., & Dühnforth, M. (2007). High-and low-latitude forcing of Plio-Pleistocene East African climate and human evolution. Journal of Human Evolution, 53(5), 475–486. https://doi.org/10.1016/j.jhevol.2006.12.009
Treves, A., & Palmqvist, P. (2007). Reconstructing hominin interactions with mammalian carnivores (6.0–1.8 Ma). In S. L. Gursky & K. A. I. Nekaris (Eds.), Primate anti-predator strategies (pp. 355–381). Boston, MA: Springer. https://doi.org/10.1007/978-0-387-34810-0_17
Tromborg, C. T., & Coss, R. G. (2015). Isolation rearing reveals latent antisnake behavior in California ground squirrels (Otospermophilus becheeyi) searching for predatory threats. Animal Cognition, 18, 855–865. https://doi.org/10.1007/s10071-015-0853-5Spon
Turner, A. (1990). The evolution of the guild of larger terrestrial carnivores during the Plio-Pleistocene in Africa. Geobios, 23(3), 349–368. https://doi.org/10.1016/0016-6995(90)80006-2
van Casteren, A., Sellers, W. I., Thorpe, S. K. S., Coward, S., Crompton, R. H., Myatt, J. P., & Ennos, A. R. (2012). Nest-building orangutans demonstrate engineering know-how to produce safe, comfortable beds. Proceedings of the National Academy of Sciences USA, 109(19), 6873–6877. https://doi.org/10.1073/pnas.1200902109
Villmoare, B., Kimbel, W. H., Seyoum, C., Campisano, C. J., DiMaggio, E. N., Rowan, J., & Reed. K. E. (2015). Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science, 347(6228), 1352–1354. https://doi.org/10.1126/science.aaa1343
Volkmar, F. R., & Greenough, W. T. (1972). Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science, 176(4042), 1445–1447. https://www.jstor.org/stable/1734595
Ward, C. V. (2002). Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? American Journal of Physical Anthropology, 119 (iS35) Supplement: Yearbook of Physical Anthropology, 185–215. https://doi.org/10.1002/ajpa.10185
Venkataramana, V. V., Kraft, T. S., & Dominy, N. J. (2013). Tree climbing and human evolution. Proceedings of the National Academy of Sciences USA, 110(4), 1237–1242. https://doi.org/10.1073/pnas.1208717110
Wallentin, M. (2009). Putative sex differences in verbal abilities and language cortex: A critical review. Brain & Language, 108(3), 175–183. https://doi.org/10.1016/j.bandl.2008.07.001
Werdelin, L., & Lewis, M. E. (2001). A revision of the genus Dinofelis (Mammalia, Felidae). Zoological Journal of the Linnean Society, 132, 147–258. https://doi.org/10.1006/zjls.2000.0260
Werdelin, L., & Lewis, M. E. (2005). Plio-Pleistocene Carnivora of eastern Africa: Species richness and turnover patterns. Zoological Journal of the Linnean Society, 144, 121–144. https://doi.org/10.1111/j.1096-3642.2005.00165.x
Werdelin, L., Lewis, M. E., & Haile-Selassie, Y. (2014). Mid-Pliocene Carnivora from the Woranso-Mille Area, Afar Region, Ethiopia. Journal of Mammalian Evolution, 21, 331–347. https://doi.org/10.1007/s10914-013-9250-5
Wong, J., Webster, M. J., Cassano, H., & Weickert, C. S. (2009). Changes in alternative brain-derived neurotrophic factor transcript expression in the developing human prefrontal cortex. European Journal of Neuroscience, 29(7), 1311–1322. https://doi.org/10.1111/j.1460-9568.2009.06669.x
Wood, B., & Lonergan, N. (2008). The hominin fossil record: taxa, grades and clades. Journal of Anatomy, 212(4), 354–376. https://doi.org/10.1111/j.1469-7580.2008.00871.x
Zisenwine, T., Kaplan, M., Kushnir, J., & Sadeh, A. (2013). Nighttime fears and fantasy-reality differentiation in preschool children. Child Psychiatry & Human Development, 44, 186–199. https://doi.org/10.1007/s10578-012-0318-x
Acknowledgements
This research was funded by Faculty Grant D922 and undergraduate teaching support from the University of California, Davis. The following individuals participated in interviewing preschool children: Monica Doherty, Patricia Fuller, Cathleen Hunt, Michelle Lollock, Doreen Kushida, Jacqueline Marnach, Shonda Miller, Stephanie Rocha, Anne Marie Stokes, and Jin-Jin Wu.
Author information
Authors and Affiliations
Contributions
This is a single-authored manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The current research was approved by Human Subjects protocols HSRC 91-243 and 93-321R from the University of California, Davis.
Consent to Participate
Parents of children and adult participants signed permission forms.
Consent for Publication
The author grants the publisher permission to publish this manuscript.
Conflict of Interest
The author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coss, R. Something Scary Is Out There: Remembrances of Where the Threat Was Located by Preschool Children and Adults with Nighttime Fear. Evolutionary Psychological Science 7, 239–253 (2021). https://doi.org/10.1007/s40806-021-00279-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40806-021-00279-9