Abstract
Background
Both ceftaroline and daptomycin are possible therapeutic options for diabetic foot infection (DFI) and both are active against methicillin-resistant Staphylococcus aureus (MRSA) infection; however, no previous studies have evaluated their effectiveness head-to-head.
Objective
This study compared hospital readmission and mortality proportions among patients receiving ceftaroline fosamil or daptomycin for DFI.
Patients and Methods
This was a retrospective cohort, comparative effectiveness study of adults (aged ≥ 18 years) admitted to United States Veterans Health Care System hospitals with a diagnosis code for DFI between 1 October 2010 and 30 September 2014 with an electronic order for ceftaroline or daptomycin as first-line therapy within 14 days of admission. Baseline characteristics were compared using Chi-square, Fisher's exact, and Wilcoxon rank-sum tests. Hospital readmission and patient mortality proportions were compared through multivariable logistic regression models with Hispanic ethnicity, prior hospitalization, dyslipidemia, and Charlson comorbidity score as covariates.
Results
In total, 223 patients were included (ceftaroline, n = 71; daptomycin n = 152). At baseline, ceftaroline patients were more likely to be Hispanic (18 vs. 6%, p < 0.01) and have been hospitalized in the past 90 days (34 vs. 19%, p = 0.02). Unadjusted 90-day hospital readmission proportions for ceftaroline versus daptomycin were 34 vs. 49%, and unadjusted 90-day mortality proportions were 1% vs. 8%. In multivariable models, ceftaroline patients were less likely to experience 90-day hospital readmission (odds ratio [OR] 0.46, 95% confidence interval [CI] 0.25–0.85) and 90-day mortality (OR 0.14, 95% CI 0.01–0.77).
Conclusions
In this population, ceftaroline was associated with lower 90-day hospital readmission and 90-day mortality compared with daptomycin when used as first-line therapy for DFI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This was a retrospective comparative effectiveness study of patients with diabetic foot infections. |
Ceftaroline was associated with fewer hospital readmissions compared with daptomycin. |
Ceftaroline was associated with lower mortality compared with daptomycin. |
1 Introduction
Up to 25% of patients with diabetes will experience a diabetic foot infection (DFI) during their lifetime [1]. DFIs are difficult to treat due to limited antibiotic penetration, frequent polymicrobial infection, and an increased incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection. Currently, the FDA has indicated only three antibiotics for the treatment of DFIs (piperacillin and tazobactam, ertapenem, and linezolid), but many others are used for treatment off-label [2]. The Infectious Diseases Society of America (IDSA) recommends empiric therapy based on infection severity and any recent microbiological data [2]. Currently, there is not a preferred therapeutic regimen that offers a significant benefit over others [3].
Gram-positive bacteria are the most common infectious organisms and MRSA is implicated in up to 17% of DFIs [4]. Empiric coverage against MRSA requires antimicrobial agents that are both effective for the treatment of DFIs and active against this pathogen. The 2012 IDSA guidelines recommend starting empiric therapy with an anti-MRSA agent in areas of high prevalence, for severe infections, and in patients with a history of MRSA infection or colonization [2]. Vancomycin is considered a mainstay treatment option to empirically cover MRSA, but daptomycin and linezolid are also suggested. For DFIs where polymicrobial infection is not suspected, monotherapy with one of these anti-MRSA agents is recommended. Vancomycin has reported success proportions of 69–73% in treating DFIs, while daptomycin and linezolid show proportions of 66–91% and 76–87%, respectively [5,6,7,8,9].
Ceftaroline was specifically omitted from the 2012 IDSA guidelines since it was approved on the basis of studies that excluded patients with DFIs. Ceftaroline studies in patients with complicated skin and skin structure infections, excluding DFIs, demonstrated success proportions of 92% [10, 11]. Since publication of the 2012 guidelines, ceftaroline has seen more widespread use for off-label treatment of DFIs, and subsequent studies have evaluated ceftaroline’s efficacy specifically in this situation. Ceftaroline was found to achieve an overall success proportion of 81% in a retrospective observational study of patients treated for DFIs, including infections involving MRSA [12]. This proportion is comparable with that reported for currently recommended therapies, but no direct head-to-head comparisons between ceftaroline and these therapies for the treatment of DFIs have been performed to date. In addition, no studies to date have evaluated mortality or hospital readmission proportions using ceftaroline to treat DFIs. A direct head-to-head comparison with current treatments and additional outcomes data may provide insight into the suitability of ceftaroline’s use for this indication and prompt reconsideration of ceftaroline’s place in guideline recommendations.
Both ceftaroline and daptomycin are possible therapeutic options for DFIs and both are active against MRSA; however, no previous studies have evaluated their effectiveness head-to-head. Ceftaroline has added gram-negative coverage compared with daptomycin. This difference could be important from an efficacy standpoint as well as an antimicrobial stewardship standpoint. A direct comparison of clinical outcomes is needed to establish whether ceftaroline is an acceptable recommendation for the empiric treatment of DFIs. This study compared 90-day hospital readmission proportions and mortality proportions for patients with DFIs who received ceftaroline or daptomycin as first-line therapy.
2 Methods
2.1 Data Source
Data were obtained from the Veterans Health Administration (VHA) electronic medical record (EMR), which includes administrative, clinical, laboratory, and pharmacy data. The VHA EMR is linked between all US-based VHA sites. The data compiled for this study thus represent nationwide VHA use of ceftaroline and daptomycin within the study period. The Institutional Review Board of the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development Committee approved this study.
2.2 Study Design
This was a retrospective cohort, comparative effectiveness study of adults aged ≥ 18 years in the US VHA with a diagnosis code for DFI during their hospital stay between 1 October 2010 and 30 September 2014.
Variables were determined prior to study initiation and included patient characteristics (age, race, Hispanic ethnicity, comorbidities, prior medications, and concomitant medications), treatment setting, the exposures of interest (ceftaroline and daptomycin use), and treatment outcomes (length of stay, 90-day hospital readmission, and 90-day patient mortality). International Classification of Diseases, Ninth Revision (ICD-9) and Clinical Modification Diagnosis (CSS) codes were utilized to identify patients with DFI and comorbidities (see electronic supplementary material [ESM] 1 and 2).
2.3 Inclusion and Exclusion Criteria
This study was designed to assess the effectiveness of ceftaroline and daptomycin as first-line therapy for patients with DFI. Patients hospitalized within the study period with a diagnosis of DFI and an electronic order for ceftaroline or daptomycin within 14 days of hospital admission were included in the study. Patients with pneumonia were excluded from this study because daptomycin interacts with pulmonary surfactant, rendering it suboptimal for treatment of patients with concomitant pneumonia. Patients who received both drugs were excluded from the study.
2.4 Statistical Analysis
Two-way statistical tests, including the Chi-square, Fisher’s exact, Student’s t, and Wilcoxon rank-sum tests, were used to compare baseline characteristics of patients treated with each agent. Baseline characteristics found to be significantly different (p ≤ 0.05) with two-way statistical tests were selected as covariates for the multivariable models. Multivariable analyses were conducted to account for the effects of dissimilar baseline characteristics between treatment arms. JMP Pro 12.1.0 (SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses.
3 Results
A total of 223 patients met the study criteria (ceftaroline, n = 71; daptomycin, n = 152). Compared with daptomycin patients, ceftaroline patients were similar in median (interquartile range [IQR]) age [61 years (57–66) vs. 60 years (55–63), p = 0.23], were more likely to be Hispanic (18 vs. 6%, p = 0.0043), had a higher proportion of prior hospitalizations in the last 90 days (34 vs. 19%, p = 0.02), and were more likely to have peptic ulcer disease (6 vs. 1%, p = 0.02) [see Table 1 for a full comparison of all baseline characteristics]. Median (IQR) time from admission to drug initiation was 0 (0–1) days for ceftaroline and 1 (0–1) day for daptomycin.
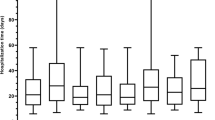
Regarding patient outcomes (Fig. 1), the unadjusted 90-day hospital readmission proportions for ceftaroline and daptomycin were 34 vs. 49% (odds ratio [OR] 0.54, 95% confidence interval [CI] 0.30–0.97; p = 0.04). Unadjusted 90-day mortality proportions were 1 vs. 8% (OR 0.17, 95% CI 0.02–1.31; p = 0.05). In multivariable models with all divergent baseline characteristics included as covariates (Fig. 2), patients treated with ceftaroline were less likely to experience 90-day hospital readmission (OR 0.46, 95% CI 0.25–0.85) and 90-day mortality (OR 0.14, 95% CI 0.01–0.77) than those treated with daptomycin.
4 Discussion
In this retrospective assessment of outcomes following real-world use of ceftaroline and daptomycin within the VHA for DFI, multivariable models demonstrated fewer hospital readmissions and lower mortality with ceftaroline versus daptomycin.
Daptomycin is a guideline-recommended, empiric treatment option for DFIs with suspected MRSA involvement. To our knowledge, this is the first direct comparison of ceftaroline with another guideline-recommended antibiotic for the treatment of DFIs. The results of this comparison support the use of ceftaroline for this purpose.
As previous studies established, clinical cure proportions of ceftaroline and daptomycin for DFIs were within the same range of each other (81% for ceftaroline, 66–91% for daptomycin) [5,6,7, 12]. Clinical cure proportions for other guideline-recommended therapies for suspected MRSA involvement were also comparable (69–73% for vancomycin, 71–87% for linezolid) [5, 8, 9]; however, previous studies did not collect data on readmission or mortality proportions. These outcome data are important from an economic and clinical perspective. Hospital reimbursement for Medicare patients depends on readmission proportion, and developing a DFI is a known, independent risk factor for mortality [13].
Currently, readmissions and mortality data for individual antibiotics in the treatment of DFI are widely unavailable. Comparisons with vancomycin and linezolid in these categories are therefore not possible at this time. However, these data for the treatment of DFIs overall have been collected. A single-center study of patients enrolled in a multidisciplinary diabetic foot service at Johns Hopkins found the overall unplanned 30-day readmission proportions for patients treated for DFI to be 17% [14]. A multicenter study of state inpatient hospitals and emergency departments in Florida and New York found 30-day readmission proportions of 30% [15]. The 90-day readmission proportions in our study of VHA patients were found to be 34% for ceftaroline and 49% for daptomycin. However, different treatment settings, lack of stratification by first-line antibiotic in prior studies, and varying time frames of reported proportions make it impossible to compare the readmission outcomes observed in this study with those of different therapy options identified in previous studies.
In addition to readmission proportions, this study also analyzed 90-day mortality proportions. Developing a DFI is a known, independent risk factor for mortality. The 5-year mortality proportion of patients with diabetic foot ulcers was estimated to be approximately 40%, but proportions could vary from 26 to 63% [16]. Data on 90-day mortality proportions involving DFIs were most often reported for patients who underwent amputation due to more severe infections and underlying disease. These proportions are not directly comparable with those found in our study for antibiotic treatment with ceftaroline or daptomycin. As with readmission outcomes, current literature does not exist to compare mortality outcomes of ceftaroline or daptomycin with other guideline-recommended therapies. While additional data are necessary for a more thorough evaluation of other therapies, this retrospective VHA study has established that first-line ceftaroline exhibited significantly lower 90-day readmission proportions and 90-day mortality proportions compared with first-line daptomycin.
A previous systematic review did not find a significant benefit associated with any single antibiotic agent in the treatment of DFIs [3]. Current IDSA guidelines recommend only three treatments options for DFI with suspected MRSA involvement: vancomycin, linezolid, and daptomycin [2]. Ceftaroline has similar clinical success proportions as all three of these therapies, and significantly lower hospital readmission and mortality proportions than daptomycin [5,6,7, 12]. These conclusions support the use of ceftaroline fosamil as a first-line antibiotic for the treatment of DFI. Additional factors of safety, cost, and therapeutic burden should be included when considering ceftaroline fosamil for this purpose.
Due to the patient population served by the VHA, there are some limitations regarding external validity. First, 95% of patients included in the study were male. Moreover, baseline characteristics and comorbidities were likely to affect hospital readmission and patient mortality. In this study, patients in each treatment arm were mostly similar in baseline characteristics and comorbidities; however, Hispanic patients were more represented in the ceftaroline treatment arm, and the ceftaroline treatment arm had a higher incidence of baseline peptic ulcer disease and prior hospitalization. To remove confounding, multivariable analyses were performed to account for differences in baseline characteristics and comorbidities. However, we were unable to account for antibiotic timing and antibiotics received prior to hospitalization, and there might be unknown confounders, such as amputation, that affect the measure of association. Due to the misbalance of individuals reporting Hispanic ethnicity between the two exposure groups, there might be an underlying difference as to why providers prescribed one therapy as opposed to the other. Furthermore, it is unknown if differences in dosing frequency, and the associated burden on nursing staff, played a role in drug selection. In addition, readmissions and mortality were all-cause, therefore underlying issues besides just DFI may have led to these negative outcomes. Furthermore, the study has a small sample size and the numbers of patients in each treatment arm were not equal. This was not a direct comparison, as seen in randomized controlled trials. Nevertheless, these real-world data are useful for clinical decision making. Finally, local prescribing patterns and drug availability likely impacted drug selection.
In spite of its limitations, our study also has notable, important strengths. This study has a larger population than previous studies. To date, it is the largest comparative effectiveness study of ceftaroline and daptomycin in the real-world treatment of DFI. The VHA is the largest integrated health care system in the US, with facilities in all 50 states. The VHA EMR data consist of clinical, pharmacy, and administrative data. These repositories are a comprehensive source for evaluating mortality, even when it occurs outside the hospital.
Further studies are needed with a larger patient population, a greater proportion of female patients, and an equal distribution of ethnic backgrounds. A network meta-analysis could provide additional useful information. Randomized controlled trials can be conducted to reduce bias and confounding factors.
5 Conclusions
This real-world effectiveness study of patients with DFI demonstrates that first-line ceftaroline is associated with lower proportions of 90-day hospital readmission and lower 90-day patient mortality than first-line daptomycin. While this study provided preliminary information on the use of ceftaroline and daptomycin for DFI, these findings must be confirmed or refuted in a larger, more diverse patient population.
References
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–28.
Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–73.
Peters EJ, Lipsky BA, Berendt AR, Embil JM, Lavery LA, Senneville E, et al. A systematic review of the effectiveness of interventions in the management of infection in the diabetic foot. Diabetes Metab Res Rev. 2012;28(Suppl 1):142–62.
Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019;56(8):907–21.
Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J Antimicrob Chemother. 2005;55(2):240–5.
Johnson K, Lamp KC, Friedrich LV. Retrospective review of the use of daptomycin for diabetic foot infections. J Wound Care. 2009;18(9):396–400.
Joseph WS, Quast T, Cogo A, Crompton MG, Yoon MJ, Lamp KC, et al. Daptomycin for methicillin-resistant Staphylococcus aureus diabetic foot infections. J Am Podiatr Med Assoc. 2014;104(2):159–68.
Lipsky BA, Itani K, Norden C, Linezolid Diabetic Foot Infections Study G. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. 2004;38(1):17–24.
Lipsky BA, Itani KM, Weigelt JA, Joseph W, Paap CM, Reisman A, et al. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus: results from three randomized controlled trials. Int J Infect Dis. 2011;15(2):e140–6.
Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculik T, et al. CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65(Suppl 4):iv41–51.
Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T, et al. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65(Suppl 4):iv53–65.
Lipsky BA, Cannon CM, Ramani A, Jandourek A, Calmaggi A, Friedland HD, et al. Ceftaroline fosamil for treatment of diabetic foot infections: the CAPTURE study experience. Diabetes Metab Res Rev. 2015;31(4):395–401.
Martins-Mendes D, Monteiro-Soares M, Boyko EJ, Ribeiro M, Barata P, Lima J, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complicat. 2014;28(5):632–8.
Holscher CM, Hicks CW, Canner JK, Sherman RL, Malas MB, Black JH 3rd, et al. Unplanned 30-day readmission in patients with diabetic foot wounds treated in a multidisciplinary setting. J Vasc Surg. 2018;67(3):876–86.
Remington AC, Hernandez-Boussard T, Warstadt NM, Finnegan MA, Shaffer R, Kwong JZ, et al. Analyzing treatment aggressiveness and identifying high-risk patients in diabetic foot ulcer return to care. Wound Repair Regen. 2016;24(4):731–6.
Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J. 2016;13(5):892–903.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by TEF-IT-41, an investigator-initiated grant to Dr. Frei’s institution, from Actavis Pharmaceuticals (formerly Forest and Allergan Pharmaceuticals). Dr. Frei was supported in part by an NIH Clinical and Translational Science Award (UL1 TR002645) during part of the time during which this study was conducted. This material is the result of work supported with resources and the use of facilities at the South Texas Veterans Health Care System. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health. The authors would like to thank student pharmacist Courtney Baus for her editorial assistance with the manuscript.
Conflict of interest
This work was supported by TEF-IT-41, an investigator-initiated grant to Dr. Frei’s institution, from Actavis Pharmaceuticals (formerly Forest and Allergan Pharmaceuticals). Alyssa C. Eaves, Chengwen Teng, and Kirk E. Evoy declare no potential conflicts of interest or competing interests.
Ethics approval
The Institutional Review Board of the University of Texas Health Science Center at San Antonio and the South Texas Veterans Health Care System Research and Development committee approved this study.
Consent to participate
Not applicable.
Consent for publication
All authors consent to the publication of this manuscript.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code that supports the findings of this study is available from the corresponding author upon reasonable request.
Author contributions
Study concept and design: CRF. Statistical analysis: CRF. Interpretation of data: All authors. Drafting of the manuscript: ACE, CT, KEE, CRF. Critical revision of the manuscript for important intellectual content: All authors. Study supervision: CRF. All authors read and approved of the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eaves, A.C., Teng, C., Evoy, K.E. et al. Retrospective Cohort Evaluating the Comparative Effectiveness of Ceftaroline and Daptomycin as First-Line Therapies for Inpatient Treatment of Diabetic Foot Infection in the United States Veterans Health Care System. Drugs - Real World Outcomes 9, 609–615 (2022). https://doi.org/10.1007/s40801-022-00319-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-022-00319-1