Abstract
Background
The role of the clinical pharmacist within the healthcare system remains unclear.
Objective
Our objective was to describe a pharmacist’s comprehensive geriatric assessment (pCGA) at admission of elderly patients and to assess its relevance in terms of medication compliance and pharmacist interventions (PIs).
Methods
We conducted a prospective interventional study over 29 months in a 34-bed medical/rehabilitation geriatric ward in a French geriatric hospital. At admission, patients received pharmaceutical care through a consistent three-step process: (1) pharmacists met with the patient to undertake cognitive screening and assess their medication adherence (using the Girerd score) and medication history; (2) medication reconciliation was conducted at admission to detect intentional and unintentional discrepancies in treatment; and (3) clinical medication review was carried out throughout the patient’s stay. The pharmacist conveyed proposed interventions to optimise treatment to the physician through the electronic health record. The number and type of PIs and their rate of implementation were recorded.
Results
In total, 539 patients aged >65 years were included; their mean age was 84 years. Cognitive screening showed that 45% of patients were confused at admission. Medication adherence assessment indicated that 50.2% had adherence problems. Medication reconciliation at admission detected discrepancies in 48%, with a mean of 1.09 unintended discrepancies per patient. Patients were taking an average of 7 ± 3 drugs. In total, 828 PIs were reported to physicians; 520 were accepted and implemented (62.8% acceptance rate).
Conclusion
This approach helps to avoid medication errors and enables the suggestion of relevant PIs, which were implemented by physicians in two-thirds of cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Elderly patients are at risk of adverse drug events, and medication errors can often occur during times of transition in care, such as admission to hospital. |
Involving clinical pharmacists in the patient management process helps to obtain more exhaustive and accurate information regarding the patient’s medication history through medication reconciliation. |
A systematic approach to pharmaceutical care at hospital admission can help identify relevant pharmacist interventions and may reduce unintended medication discrepancies. |
1 Introduction
Many elderly people living at home have multiple medical conditions [1] and consequently require multiple drugs on a daily basis [2]. Polypharmacy is particularly prevalent in France; a recent study observed polypharmacy (defined as five to nine drugs) in 53.6% of a population of 2350 patients aged ≥70 years living at home, and excessive polypharmacy (ten or more drugs) in 13.8% [3]. Age-related pharmacokinetic and pharmacodynamic changes make older people more susceptible to the risk of iatrogenic complications [4, 5]. In addition, elderly individuals are also exposed to the risk of adverse drug events in the hospital setting, and these adverse events may contribute to prolonged hospitalisation and additional costs [6–8].
Several specific problems are associated with geriatric prescription, including a lack of data from clinical drug trials, lack of pharmacotherapy management in the elderly patient, ageism, and poor communication between prescribers [9, 10]. These factors may lead to inappropriate prescriptions in this population [11, 12].
In this context, various criteria for the appropriate management of drug prescriptions in elderly patients have emerged [13, 14], and pharmacists have been closely involved in the development and implementation of these tools. The effective use of clinical pharmacy services has been shown to reduce mortality and costs [15, 16]. In France, only medication review is clearly defined as within the pharmacist’s responsibilities. Christensen and Lundh found that medication review might reduce emergency department contacts [17]. The use of medication reconciliation (MR) has been developing recently, particularly in care of the elderly. This is a process whereby the most accurate list possible of all medications received by a patient is created—it has been shown to detect between 0.4 and 2.13 unintentional discrepancies (UIDs; involuntary differences between the previous treatment and the admission treatment, either because the prior treatment was unknown or because of an error during prescription) in treatments in 24–82% of patients at admission at emergency service and acute care hospitals [18–20].
Although models for involving clinical pharmacists in the patient management process exist, in France the role of the pharmacist within the healthcare system remains unclear [21–23].
Involving clinical pharmacists in the care process in our geriatric hospital has helped to define a coherent pharmaceutical care process. Hepler and Strand [24] defined pharmaceutical care as the responsible provision of drug therapy to achieve definite outcomes that improve a patient’s quality of life.
The structured implementation of clinical pharmacy activities led to the creation of the pharmacist comprehensive geriatric assessment (pCGA), which is now performed routinely when elderly patients are admitted to our institution and during hospitalization. This approach to pharmaceutical care enables a holistic approach to patient care as well as the identification of numerous drug-related problems (DRPs; defined as an event or circumstance involving drug therapy that actually or potentially interferes with the desired health outcome).
In this context, the aims of this study were to (1) describe the pharmaceutical care process (termed pCGA) that is performed upon admission of elderly patients and (2) evaluate the relevance of the routine use of pCGA by assessing cognitive screening, medication adherence, MR at admission, rate and number of pharmacist interventions (PIs) proposed, and implementation of these PIs.
2 Methods
2.1 Design
This was a 29-month prospective observational study carried out from 29 November 2011 to 6 February 2014 at the Bertinot Juël Geriatric Hospital, in Chaumont En Vexin in the north of France. This local hospital dedicated to geriatric care comprises a 34-bed medical/rehabilitation ward and an 86-bed long-term care unit, and also conducts outpatient consultations. The inclusion criteria for the study were patients aged at least 65 years who were taking at least one drug at admission and who were admitted to the medical/rehabilitation ward. We excluded patients aged <65 years, those with no prescribed medication at admission and patients admitted to the long-term care unit.
2.2 Pharmacist Comprehensive Geriatric Assessment (pCGA) Development and Implementation
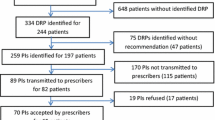
Over several months, in collaboration with the interdisciplinary team that included geriatric medicine specialists, nurses and clinical pharmacists, we developed a procedure we termed ‘pharmacist’s comprehensive geriatric assessment’ (pCGA) that was to be performed systematically upon admission of all elderly patients to our institution. This geriatric pharmaceutical care process consists of three steps (described below and in Fig. 1) and was carried out by a pharmacist, a resident or a pharmacy student depending on the available personnel. A senior pharmacist trained all residents and students on how to perform the pCGA at the beginning of their placement in our institution. A resident in pharmacy is a postgraduate who has studied pharmacy for 5 years to become a hospital pharmacist. The residency lasts 4 years. All the residents involved in this study were at least in their second year of residency. They were supervised by a senior pharmacist only in the first month; the pharmacy students were always supervised.
Prescription and patient records (using Osiris software 2.21, Corwin, Villers-Bretonneux, France) were consulted at the pharmacy every morning during the working week to identify new admissions and collect the following variables in a dedicated case report form: patient socio-demographic information, admitting department, reason for admission and drug prescription. For admissions during weekends or holidays, the pCGA was conducted on the next working day. The following three steps of the pCGA were implemented:
2.2.1 Step 1: Patient Assessment
The clinical pharmacist (or resident or pharmacy student) met with the patient within 24 h of admission, or on the next working day for patients admitted at the weekend or on holidays. The meeting with the patient began with cognitive screening. Since our evaluation included an assessment of medication adherence, self-medication and treatment management, we had to first ensure that the patient was not confused, which would invalidate their responses. Therefore, geriatric medicine physicians and the pharmaceutical and rehabilitation teams developed a short cognitive screening tool specifically for this study that could be implemented easily by all staff so we could rapidly rule out confusion. This test was called the spatial–temporal orientation test (STOT) and comprised only four questions that solicited long-term and recent memory: what year is it; what city is this; where do you live; and is it morning or afternoon? If the patient could not answer at least three questions correctly, they were considered confused and we did not assess medication adherence.
If the patient was considered oriented, we continued with the Girerd score, which is a six-item questionnaire derived from the Morisky Medication Adherence scale; it investigates medication adherence on the basis of the patient’s self-reported answers (Table 2) [25]. One point is given for each ‘yes’ answer, with a score of 0 indicating good adherence, a score of 1–2 indicating minimal adherence problems, and a score of ≥3 indicating poor adherence.
All patients were asked about their current medications, allergies, use of natural products, use of alcohol, use of tobacco, self-medication and the address of their local community pharmacy.
2.2.2 Step 2: Medication Reconciliation at Admission
At admission, a MR was performed for all patients; this comprised the collection and accurate identification of the patient’s current list of medications. This list was then compared with the medication prescribed at admission by the physician on duty. Possible sources used for the MR included the patient’s local community pharmacy, the patient themselves, a previous prescription, medical records from prior hospital admissions (in our institution or elsewhere), nursing home liaison forms (for patients transferred from a nursing home), the patient’s general practitioner (GP), the family, a letter of referral from the GP, examination of the patient’s treatment (for patients who presented with their ongoing treatment) and specialists from other disciplines. We telephoned the community pharmacist, who then faxed the patient’s medication list. We consulted as many sources as needed (according to availability) to obtain accurate information.
By comparing the patient’s prior medication history and the admission prescription, we were able to identify and record both intentional discrepancies (IDs), defined as voluntary discrepancies, justification for which was not documented in the patient’s record, and UIDs, as defined in the introduction. UIDs included an omission from or addition to the treatment, an adjustment to the dose or dosage and wrongful substitution. IDs and UIDs were classified after referring back to the prescribers.
2.2.3 Step 3: Medication Review and Implementation of Pharmacist Interventions
We conducted a clinical medication review, including clinical, biological and MR data [26]. After the in-depth review and MR at admission, the effectiveness and patient tolerance of drug therapy were followed throughout the patient’s hospitalization. Our review included STOPP (Screening Tool of Older People’s Potentially Inappropriate Prescriptions) and START (Screening Tool to Alert doctors to Right Treatments) criteria, the Beers criteria, the presence of a prescription cascade, evaluation of renal function according to creatinine clearance and dosage adjustment according to biological data [13, 14]. We identified and recorded any DRPs [27]. We used a validated French-language instrument to classify DRPs into the following categories: untreated indication, supratherapeutic dosage, non-indicated drug, non-compliance with guidelines/contra-indication, drug monitoring, sub-therapeutic dosage, adverse drug reaction, improper administration, drug interaction and failure to receive drug in the presence of a clear indication [28]. Depending on the findings of this review, the pharmacist proposed one or several PIs to improve the quality of the patient’s pharmacotherapy. PIs were emailed via the prescription system’s messaging system to the physician along with a summary of the review and the proposals for intervention, and the summary of the assessment was also recorded in the patient’s medical file. The physician decided whether or not to implement the PI. We rescreened the patient records after 2 days to ascertain whether the PIs were implemented.
2.3 Statistical Analysis
Quantitative data are described as mean ± standard deviation, or median (range) for normally distributed and non-normally distributed variables, respectively. Age was classified into four categories (65 to <80 years, 80–84 years, 85–89 years, and ≥90 years); the number of drugs being taken was classified into three categories (≤3, 4–12, and ≥13). Quantitative variables were compared using the Student’s t or Mann–Whitney tests, and qualitative variables were compared using the chi squared or Fisher’s exact test, as appropriate. A p value of <0.05 was considered statistically significant. All analyses were performed using STATA 10.1 software (Stata Corp., College Station, TX, USA).
3 Results
Among 1035 admissions between 29 November 2011 and 6 February 2014, sufficient clinical pharmacist personnel were available to perform pCGA in 600 patients (58% of admissions). Among these, 61 did not meet the inclusion criteria (Fig. 1), yielding a study population of 539 patients (Table 1). The average age was 84 ± 7.1 years; 242 (45%) of these patients were considered confused according to our ad hoc screening tool. The average length of stay was 11.5 days.
Of the 539 patients, 462 (86%) were receiving between 4 and 12 drugs, 28 (5%) were receiving at least 13 drugs, and 49 (<10%) were receiving three drugs or fewer. Of note, 137 (25.4%) were receiving more than ten drugs. Confused patients took significantly fewer drugs than oriented patients (p = 0.010) (Table 1).
We used the Girerd score in the 297 oriented patients. Slightly more than half of these patients had adherence problems. We found a borderline significant relationship between age and adherence, with older patients tending to have better adherence (p = 0.05). Table 2 presents the responses to the Girerd score among oriented patients.
We conducted MR at admission for all patients, and the number of sources of information ranged from one to five (average of three; Table 3). Overall, there were 588 UIDs, with an average of 1.09 discrepancies per patient. At least one UID was observed in 260 of 539 patients (48%). There was a significant relationship between the number of UIDs identified and the number of sources of information used to perform MR (p = 0.002).
Table 4 details the number of PIs by intervention outcome, type of DRP and type of PI as well as listing the ten drugs most commonly involved in the PIs. The Anatomical Therapeutic Chemical (ATC) classification code for the medicines cited in the 828 PIs were as follows: cardiovascular system (243 [29%]), nervous system (200 [24%]), blood and blood-forming organs (129 [16%]) and alimentary tract and metabolism (123 [16%]) [29].
Medication review at admission and during hospitalisation led to 828 PIs being proposed. Approximately two-thirds of these were implemented by the physician (520 PIs, for a physician acceptance rate of 62.8%). For the 308 PIs that were not implemented, 120 (39%) were refused and 188 (61%) were not evaluated because the pharmacist’s notes were not seen by the physician.
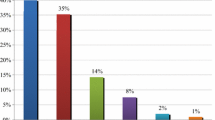
We recorded five primary types of PIs; dose adjustment was the most frequent, at 28% (233/828), predominantly a recommendation that a dose be adjusted to account for renal function. Addition of a new drug represented 23% (187/828) of PIs, covering non-renewal of outpatient treatment at admission or treatment for untreated indications. A total of 22.5% (186/828) related to drug discontinuation, covering recommendations to discontinue treatments prescribed at admission by error, drugs prescribed in the absence of an indication or even inappropriate medication. Drug switches accounted for 12% (99/828) of PIs and were recommended for medical reasons (and not because of lack of availability, for example).
4 Discussion
This study aimed to describe our pCGA procedure implemented upon admission of elderly patients and to evaluate its relevance in terms of medication adherence, MR at admission and PIs proposed to correct any UIDs. We found that more than one-half of oriented patients had adherence problems, MR revealed an average of at least one UID per patient, and approximately two-thirds of all PIs were implemented by the physicians.
Structuring pharmaceutical care at admission can help to ensure the reproducibility of pharmaceutical management and contribute to greater transparency in the process of care. However, delivery of pharmaceutical care on a systematic basis, for example through MR at admission, can be difficult to achieve, because it is largely dependent on the availability of pharmaceutical staff. In our study, we had sufficient staff to perform the pCGA for 58% (600/1035) of all admissions. Patients also received follow-up during hospitalization.
In our study, we screened for cognitive function using the ad hoc STOT instrument as the first step of the pCGA. Although this tool is easy and quick to implement without any training, it is not a validated instrument. However, it allowed us to identify rapidly whether we would be able to perform a useful evaluation of treatment compliance with the patient. Even evaluating compliance only among those considered not to be confused, we observed adherence results close to those reported by the World Health Organisation, with approximately one-half of patients in the study presenting at least minimal non-compliance [30]. Krousel-Wood et al. [31] also found that the adherence of 55% of elderly patients (aged 75 ± 5.6 years) was low. The Girerd score, used to evaluate compliance, is a self-reported method and therefore has some limitations. For example, it does not account for adherence problems specific to the elderly, such as cognitive impairment or dexterity problems. However, it is one of the few validated tools in the French language to evaluate level of adherence. We observed that the older the patient, the better the compliance. One potential explanation for this finding is that older patients may have caregivers or home help who contribute to ensuring good compliance. Indeed, the World Health Organisation underscores that a prerequisite to improving adherence is the assessment of the patient’s mental status, which is why we chose to screen cognition before evaluating compliance. For patients considered unlikely to provide reliable answers to the Girerd score, treatment adherence should be discussed with the family and/or caregivers.
Evidence suggests that approximately 24–60% of elderly patients have at least one UID at admission, and rates of 1–2.13 UIDs per patient have been reported [18, 20]. Our results are in line with these findings: 48% of patients in our study had at least one UID. To identify the patients’ treatments, our MR primarily involved the patient’s local pharmacist, the patients themselves, and the admission prescription. Community pharmacists are a useful source of reliable and up-to-date information and are easy to contact, usually respond immediately and provide objective data. Closer collaboration with community pharmacists, particularly during times of transition of care, could certainly improve the quality of care in the elderly population and reduce the risk of medication errors. We observed that the more sources of information that were used, the more UIDs were found. It is necessary to cross-reference a maximum of sources to obtain the most exhaustive medication information possible and, thereby, intercept a maximum of errors.
In addition to clinical and biological data, MR in our study also included a clinical medication review. This type of procedure is rarely carried out in Europe [32]. In our study, an overall total of 828 PIs were proposed for the 539 patients included, indicating that our comprehensive approach made it possible to detect a large number of errors requiring intervention. Overall, we achieved a 62.8% medical acceptance and implementation rate, while 22.7% of the proposed PIs were not evaluated and only 14.5% were declined by the physician. These figures are consistent with those in previous literature (50–98%) [33–37]. To improve the rate of PI acceptance by physicians, it might be fruitful to share the non-evaluated PIs (almost one-quarter of all PIs) with the prescribers, either by telephone or in direct face-to-face contact in the department. Indeed, verbal interventions have been shown to have a higher acceptance rate than written procedures [33, 34]. In addition, interventions that cannot be performed immediately could be transmitted to the GP at discharge for consideration at a later date. We identified five primary types of PIs, namely dose adjustment, addition of a new drug, drug discontinuation, drug monitoring and drug switching. Unfortunately, the clinical impact of our interventions was not evaluated in this study, and this represents an interesting target for future research.
Admission of elderly patients to hospital represents a critical transition of care, and our pCGA performed at admission helps reduce the potential for medication errors during this transition. Patients also require specific care at discharge, which is another important time of transition, and discharge care should comprise MR associated with patient therapeutic education. However, this represents a significant workload and is largely dependent on the availability of enough pharmaceutical staff. Therefore, the development of clinical pharmacy services is critical to achieving wider implementation of our pCGA process.
5 Conclusion
pCGA, as performed in our study, promotes a systematic approach to pharmaceutical care processes upon admission of elderly patients to hospital. It comprises a global approach to the patient but requires full integration of the clinical pharmacist into the multidisciplinary medical team and availability of sufficient staff. Pharmaceutical evaluation means we can enhance patient safety at times of transition in care and may reduce the potential for error through PIs. Further studies are required to evaluate the impact of PIs on clinical outcomes.
References
Tinetti M, Fried T, Boyd C. Designing health care for the most common chronic condition: multimorbidity. JAMA. 2012;307:2493–4.
Maher R, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65.
Herr M, Robine JM, Pinot J, et al. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015;24(6):637–46. doi:10.1002/pds.3772.
Mangerel K, Armand-Branger S, Rhalimi M. Clinical pharmacist and geriatric syndromes. J Pharm Clin. 2014;33:7–19.
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and pratical aplications. Br J Clin Pharmacol. 2004;57:6–14.
Naples J, Hanlon J, Schmader K, et al. Recent literature on medication errors and adverse drug events in older adults. J Am Geriatr Soc. 2016;64(2):401–8. doi:10.1111/jgs.13922.
Salvi F, Marchetti A, D’Angelo F, et al. Adverse drug events as a cause of hospitalization in older adults. Drug Saf. 2012;35(Suppl 1):29–45. doi:10.1007/BF03319101.
Doucet J, Jego A, Noel D, Geffroy CE, et al. Preventable and nonpreventable risk factors for adverse drug events related to hospital admissions in the elderly: a prospective study. Clin Drug Invest. 2002;22:385–92.
Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine”. Am J Med. 1997;103:529–35.
Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ. 2003;326:196–200.
Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and opimised? Lancet. 2007;370:173–84.
Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370:185–91.
O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246.
Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy. 2007;27(4):481–93.
Bond CA, Raehl CL, Franke T. Clinical pharmacy services, pharmacy staffing, and the total cost of care in United States hospitals. Pharmacotherapy. 2000;20(6):609–21.
Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016; (2), Article No. CD008986. doi:10.1002/14651858.CD008986.pub3.
Steurbaut S, Leemans L, Leysen T, et al. Medication history reconciliation by clinical pharmacists in elderly inpatients admitted from home or a nursing home. Ann Pharmacother. 2010;44:1596–603.
Vira T, Colquhoun T, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–6.
Van den Bemt PM, van der Schrieck-de Loos EM, van der Linden C, et al. Effect of medication reconciliation on untntional medication discrepances in acute hospital admissions of elderly adults: a multicenter study. J Am Geriatr Soc. 2013;61(8):1262–8.
Thompson CA. Pharmacists integrate into geriatric emergency department. Am J Health Syst Pharm. 2015;72(2):92–4. doi:10.2146/news150007.
American Pharmacists Association and National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model. Version 2.0. March 2008. http://www.pharmacist.com/mtm/CoreElements2. Accessed 22 May 2014.
Roth MT, Ivey JL, Esserman DA, Crisp G, Kurz J, Weinberger M. Individualized medication assessment and planning (iMAP): optimizing medication use in the primary care setting. Pharmacotherapy. 2013;33:787–97.
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
Girerd X, Hanon O, Anagnostopoulos K, Ciupek C, Mourad JJ, Concoli S. Evaluation de l’observance du traitement anti-hypertenseur par un questionnaire : mise au point et utilisation dans un sevice spécialisé. Presse Med. 2001;30:1044–8.
Clyne W, Blenkinsopp A, Seal R. A guide to medication review, 2008. http://www.cff.org.br/userfiles/52%20-%20CLYNE%20W%20A%20guide%20to%20medication%20review%202008.pdf. Accessed 10 May 2015.
Strand LM, Morley PC, Cipolle RJ, et al. Drug-related problems: their structure and function. DICP. 1990;24:1093–7.
Allenet B, Bedouch P, Rose FX, et al. Validation of an instrument for the documentation of clinical pharmacist’s interventions. Pharm World Sci. 2006;28:181–8.
World Health Organisation Collaborating Centre for Drug Statistics Methodology. ATC Structure and principles. http://www.whocc.no/atc/structure_and_principles/. Accessed 25 Oct 2016.
World Health Organisation. Adherence to long-term therapies: Evidence for action. Geneva: WHO; 2003. http://whqlibdoc.who.int/publications/2003/9241545992.pdf?ua=. Accessed 9 Apr 2015.
Krousel-Wood P Muntner, Islam T, et al. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults (CoSMO). Med Clin N Am. 2009;93(3):753–69. doi:10.1016/j.mcna.2009.02.007.
Blajeva A, Labberton L, Leikola S, Pohajanoska-Mäntylä M, Geurts MME, De Gier JJ, et al. Medication review practices in European countries. Res Soc Adm Pharm. 2014;10:731–40.
Kucukarslan SN, PetersM MlynarekM, et al. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163:2014–8.
Blix HS, Viktil KK, Moger TA, et al. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci. 2006;28:152–8.
Galindo C, Olive M, Lacasa C, et al. Pharmaceutical care: pharmacy involvement in prescribing in an acute-care hospital. Pharm World Sci. 2003;25:56–64.
Guignard B, Bonnabry P, Perrier A, et al. Drug-related problems identification in general internal medicine: the impact and role of the clinical pharmacist and pharmacologist. Eur J Int Med. 2015;26:399–406.
O’Sullivan D, O’Mahony D, O’Connor MN, et al. The impact of a structured pharmacist intervention on the appropriateness of prescribing in older hospitalized patients. Drug Aging. 2014;31:471–81.
Acknowledgements
The authors thank the Regional Health Agency of Picardie, the Bertinot Juël hospital center, C. Louchet, X. Deviot, F. Lopes, E. Mannessiez, M. Charles, M. Paindavoine, C. Delarue, P. Jaecker and L. Mallet.
Author contributions
Faiza Rhalimi and Mounir Rhalimi designed the study and interpreted the results. Alain Rauss performed the statistical analyses and interpreted the results. Faiza Rhalimi wrote the manuscript. Mounir Rhalimi contributed substantially to the study concept and revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and endorse the data and conclusions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Regional Health Agency of Picardie, a regional representation of the Ministry of Health and the Bertinot Juël hospital center.
Conflict of interest
The content of this article is the sole responsibility of the authors. Faiza Rhalimi, Mounir Rhalimi and Alain Rauss have no conflicts of interest that are directly relevant to the content of this review.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rhalimi, F., Rhalimi, M. & Rauss, A. Pharmacist’s Comprehensive Geriatric Assessment: Introduction and Evaluation at Elderly Patient Admission. Drugs - Real World Outcomes 4, 43–51 (2017). https://doi.org/10.1007/s40801-016-0098-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-016-0098-x