Abstract
An open air stockpile of conical shape was formed with 3.5 m base diameter and 5 m height using 500 tons of − 200 mm size ROM thermal coal on a concrete floor. Coal sample from the stockpile were collected at 30 days interval for analysis of various chemical properties like; proximate analysis, ultimate analysis, gross calorific value (GCV) and various combustion related properties such as ignition temperature, peak temperature, burnout temperature, maximum combustion rate, ignition index, burnout index, combustion performance index and combustion rate intensity index. Experimental results show that, due to weathering of coal fixed carbon decreased from 35.6% to 19.9%, elemental carbon (C) decreased from 46.6% to 28.6%, hydrogen (H) decreased from 3.3% to 2.9% and GCV decreased by up to 55% of original value during 330 days of storage of coal in an open atmosphere. Ash content of coal increased form 29.2% to 46.6% due to loss of combustibles. Sulfur (S) of coal was found to get increased from 0.33% to 1.08% during storage. The activation energy of coal combustion increased from 22 kJ/mol to 54 kJ/mol. Variation in combustion parameters signifies that weathering has significant negative impact on coal combustion properties as coal become difficult to ignite.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
In India coal fired utilities and captive power plants are situated across the entire country. Large number of plants are located far away from the mines and ports. Due to uncertainty in availability of railway rakes for loading and transportation as well as rail movement which is often affected by various externalities that include local law and order issues, intensity of monsoon and saturation of railway network (Bhattacharaya and Maitra 2007), storage of coal in the stockpile is necessary to take care of any disruptions in the transport system and also in the coal mines. The general practice is to provide up to 2 weeks stockpile in case of a pit head utility and 30–45 days capacity (around 50,000–800,000 ton of coal) for the power plants far away from the coal mines. In some cases, coast based utilities burning imported coal have a tendency to buy large volumes of coal from the overseas market, when the price is low and then stockpile for a reasonably long period of more than 6 months. There are utilities that do open air stockpiling of coal for months, even up to 1 year. These stockpiles of about 50,000 ton each are usually formed by telescopic chute arrangement and by dozing and are mostly open type, though covered larger stockpiles are not uncommon.
Stockpiling of coal for long time causes the coal getting exposed to various oxidative conditions known as weathering of coal. The exposure of coal to that condition leads to oxidation of coal that might result in deterioration of coal’s gross calorific value (GCV), in modified coal behavior during grinding and combustion and finally in large amount of ash generation. As a result weathering of coal is a matter of great importance for the power plants. Certain work has been reported in literature on weathering of coal. Crelling et al. (1979) reported the effect of weathering on metallurgical coal where caking properties declined. Xia and Yang (2014) studied the changes in surface properties of anthracite coal before and after the weathering process and also inside and outside the weathering process. They observed that, C–C and C–H groups on the surface of anthracite coal was decreased after weathering process while the content of C–O, C=O and O=C–O groups were increased. Wagner 2008, studied the combustion behavior of weathered coal and reported that combustion performance varied between samples taken from within the same stockpile at different depths, and also between samples with different particle sizes, chemical and petrographic compositions, between weathered state and the original coals. Therefore, it can be stated that, weathering of coal largely affects the coal properties by changing the surface properties, chemical bonding between C, H, O and other elements present in coal that largely affects the coal properties. In the only major published work on Indian coal, Banerjee et al. (2000) reported coal-quality deterioration in a coal stockpile of a utility. In their study, they showed that significant amount of GCV was lost during storage. They also reported that, due to weathering, coal loose its cohesion and disintegrates into progressively smaller pieces, which may be washed away.
Most of the previous work reported the structural changes C–C and C–H bond, changes in caking properties, petrographic compositions, coke properties (Banerjee et al. 2000; Xia and Yang 2014; Cimadevilla et al. 2005; Smędowski and Piechaczek 2016; Tamamura et al. 2015; Kříbek et al. 2017) etc. in weathered coal. Quite limited study has been so far reported on the comprehensive chemical properties like; proximate analysis (PA), ultimate analysis (UA), GCV variation and finally changes in combustion behavior of weathered coal and its probable effect on working utilities. Since combustion performance of coal depends on PA, UA, GCV parameters, it is essential to know such important characteristics parameters of weathered coal stored in stockpile before burning in boiler. Therefore, the objective of the investigation was to study the effect of weathering on various coal properties like; ash, volatile matter (VM), fixed carbon (FC), carbon (C), hydrogen (H), sulfur (S), GCV of coal. Further various combustion related parameters like; ignition temperature, peak temperature, burnout temperature, maximum combustion rate, ignition index, burnout index, combustion performance index and combustion rate intensity index have been analyzed to identify the changes in combustion behavior of coal in stockpile. This study will be helpful for coal fired utilities to identify the maximum possible time coal can be kept in stockpile before major changes occur in coal properties, thereby efficiently use stockpile coal and reduce environmental impact of coal combustion.
2 Experimental
500 Tons of Run of Mine (ROM) coal of − 200 mm size were collected from Singurali coalfield located in the state of Madhya Pradesh, India. A conical shape stockpile with base diameter 3.5 m, height 5 m on concrete floor was prepared and kept in open air. Stockpile was made in the month of November i.e. end of fall and samples were collected every month (30 days interval) for analysis of coal properties. During collection of coal sample from designated points, about 15 cm depth of coal were removed from the stockpile surface to ensure that collected samples are not affected by surface abrasion. Subsequently typical channel samples of 50 kg were collected by following the ASTM D 4596-09 method, standard practice for collection of channel samples of coal in a mine. After sample preparation ASTM guideline, coal was analyzed for proximate analysis (Ash, volatile matter, moisture and fixed carbon), ultimate analysis (carbon, hydrogen, nitrogen and sulfur) using CHNSO analyzer (Vario EL III Elemental, Germany), GCV analysis using automatic bomb calorimeter (AC 350, LECO, USA). Combustion characteristics of the weathered coal were carried out using Thermo Gravimetric Analysis (TGA) in a Thermo-gravimetric Analyzer (Model: STA 449 F3 Jupiter; Make: NETZSCH) by heating the coal in the presence of oxygen gas (rate 60 ml/min) at a heating rate of 10 °C/min. By analyzing weight loss (TGA) and rate of weight loss (DTG) data points, different combustion parameters namely temperature of ignition \(T_{i}\) (°C), peak temperature \(T_{p}\) (°C) and temperature of burnout \(T_{f}\) (°C), were identified (Zhang et al. 2015). \(T_{i}\) is defined as the temperature at which a fuel shows weight loss of 1% per minute signifying the initiation of combustion process. Peak temperature \(T_{p}\) is the temperature corresponds to the maximum weight loss rate and burnout temperature \(T_{f}\) corresponding to the completion of combustion when the rate of weight loss reduces to 1 wt% per min (Ken et al. 2018). Further, from TGA–DTG data different combustion indices such as index of ignition (\(D_{i}\)), index of burn-out (\(D_{f}\)), index of combustion performance (S) and the rate and index of heat intensity during combustion process (\(H_{f}\)) were calculated in the manner shown in Eqs. (1–4) as,
where \(DTG_{ \hbox{max} }\)(wt%/min) is the peak combustion rate corresponding to \(T_{p}\), \(t_{p}\) (min) is the maximum peak combustion time \(DTG_{ \hbox{max} }\), \(t_{i}\) (min) is the time of ignition, \(\Delta t_{1/2}\) is the time range of \(DTG /DTG_{ \hbox{max} } = 0.5\), \(t_{f}\) (min) is the time of burn-out, \(DTG_{\text{mean}}\) is the mean combustion rate (mg/min), and \(T_{1/2 }\) (°C) is the temperature corresponding to occurrence of 50% burn off. Coats–Redfern method (Ebrahimi-Kahrizsangi and Abbasi 2008) was applied to estimate the Arrhenius parameters of coal combustion with assumption that reaction is of the first-order Arrhenius law using the following equation:
where α is the extent of conversion, expressed as \(\alpha = \frac{{(m_{0} - m_{t} )}}{{(m_{0} - m_{\infty } )}}\) and \(f(\alpha ) = (1 - \alpha )\). f (α) is the hypothetical model of the reaction mechanism, A is the frequency factor (min−1); E (kJ/mol) is the apparent Activation Energy; R = 8.314 kJ/mol, universal gas constant, T is the absolute temperature; m0 is the initial mass of the sample; mt is the mass of the sample at time t; m∞ is the final mass of the sample.
For combustion with constant heating rate \(\frac{{{\text{d}}\alpha }}{{{\text{d}}t}}\) can be replaced with the term \(\frac{{{\text{d}}\alpha }}{{{\text{d}}T}}\) × β, where, β = \(\frac{{{\text{d}}\alpha }}{{{\text{d}}T}}\) is the heating rate. In such case, Eq. (5) can be transformed to following form
Coats–Redfern utilized the asymptotic series expansion for approximating the exponential integral in Eq. (6) and taking logarithmic function on both the sides of Eq. (5) gives the formula for calculating the activation energy value as (Ebrahimi-Kahrizsangi and Abbasi 2008):
For hydrocarbon fuels, assuming 2RT/Ea ≪ 1, Eq. (6) can further approximated to
By plotting the appropriate left-hand side of the below equations versus 1/T or \(\left( {{ \ln }\frac{{ - \ln \left( {1 - \alpha } \right)}}{{T^{2} }}\;{\text{vs}}.\; \left( {\frac{1}{T}} \right)} \right)\). The slope equals −E/R and from there the value of E and A can be calculated from the above equations.
3 Results and discussion
3.1 Effect of weathering on ultimate analysis of coal
Figure 1a shows month by month variation of carbon content of the stockpiled coal. It is observed that carbon percentage of coal decreased from 52.5% to 34.2% during storage. There is practically no decrease in the initial period of storage with largest drop in the dry winter months followed by steady decline in the subsequent months. In the months of March–April nominal difference can be observed in morning versus evening samples. The trend observed is in agreement with a previous study carried out for a high rank coal from North Karanpura coalfield of India over a period of 8 months (Banerjee et al. 2000). Continuous decline in carbon content of the stockpiled coal should be a major cause of concern for utilities as carbon is the primary source of energy in coal and mostly contribute to the GCV, on the basis of which thermal coal is sold and bought in India. Figure 1b showing month by month variation of hydrogen content in coal indicate a decrease from 3.71% to 2.51% during storage. Other than the months of December and February, morning and evening samples show practically the same hydrogen content. On month to month basis there is however a steady decline, sharpest decrease being through the months of November to January. Continuous decline in hydrogen content of the stockpiled coal should also be a major concern for utilities as in addition to carbon, hydrogen is another source of energy in coal contributing to heat value (GCV), the basis of trading of thermal coal. Carbon and hydrogen content decline appears to be due to the heat release on wetting because of the coal stockpile exposure to alternate rain resulting humid weather and sunshine resulting dry climate. The temperature of coal bed increase due to release of heat thus advances the oxidation process. Temperature of coal bed increases progressively due to exothermic aerial oxidation process. With increase in temperature, oxidation rate increases. With fall in heat dissipation rate, spontaneous combustion of coal occurs and is generally observed for low-rank coals which when stockpiled likely to auto oxidate and lead to spontaneous heating and combustion of carbon. It has further been reported that organic content in bituminous coal decreased while the inorganic content rises during weathering process. Gas components like CO, CO2 and water are released due to oxidation of organic materials. C–C and C–H groups likely to decreased with rise in C–O, C=O and O=C–O content during weathering of coal for a period of initial 6 months. Also, the surface C/O atomic ratio decreased from 2.66 to 0.88 (Xia and Yang 2014; Cimadevilla et al. 2005). Since, nitrogen is almost entirely present in coal matter, its decomposition invariably leads to release of nitrogen as well. That explains similar trends in the decline of hydrogen and nitrogen content observed for the stockpiled coal. Figure 1c, indicates the nitrogen content decreasing from 1.32% in the month of November to 0.83% in the month of October next year. Similar to hydrogen content for the month of December (winter), morning and evening samples show some difference. Quite significant is the change in sulfur content of the coal stockpiled in open air for a period of 1 year. It increased from 0.36%, a value typical for most of the Gondwana coal in India to 1.08%, a value that exceeds the typical sulfur limit of 1% imposed on internationally traded coal (Fig. 1d). Sulfur content only nominally increased from the months of November to March. In subsequent 3 months, it kept on increasing, recording a sharp rise in the months of June to October. In coal stockpiles, pyrite oxidation is probably a factor for the cause of spontaneous heating mainly for the two reasons: (1) the reaction is highly exothermic (2) the associated coal is disintegrated during the reaction and that increases the amount of exposed reactive coal surface. Finer and more dispersed, the presence of pyrite in coal, the more effective it is in promoting self-heating. Significant dissemination of fine to ultra-finely sized mineral matter in Gondwana coal of India is well known. The dry oxidation of pyrite representing literature (Bhatt and Agarwal 1996) outline the reactions. GCV results are in agreement with ultimate analysis results for carbon, hydrogen and nitrogen (Fig. 1a–d).
Overall from Fig. 1a–d, it can be observed that, variations of carbon, hydrogen, nitrogen, sulfur are not uniform and in some cases values are lower in previous months and higher in later months. Similarly morning and evening samples have significant deviations. This can be explained by the fact that, coal is extremely heterogeneous and getting affected by weathering. Such weathering occurs due to various physico-chemical reactions at in situ of coal surface which is governed by many parameters like hydrocarbon structure of coal, mineral matter composition, moisture, coal porosity, rainfall, fog etc. Hence, rate of oxidation of coal is not uniform for each coal particles. Also higher the temperature, greater is the rate of oxidation and heat buildup. Whether fire occurs or not, the temperature increases and progressive reaction with atmospheric oxygen gradually impairs certain properties of coal. Coal oxidation leads to chemisorption of oxygen at the coal surface along with formation of acid functional groups such as hydroxyl, carboxyl and other that leads to subsequent thermal decomposition and decrease in the aliphatic and alicyclic carbon and hydrogen content. Hence results of morning and evening samples, month by month may deviate significantly. However, to minimize such errors in results, collections of higher no’s of samples are necessary. In the present study two different samples from same locations during morning and evening were collected and average value are reported. The average value for each month reports the decline in coal properties values across different months of year.
3.2 Effect of weathering on proximate analysis of coal
Proximate analysis in duplicate on as received basis across the year for all the samples were carried out. Further, average results of the same on month to month basis for all the morning and evening coal samples across the year are presented in Fig. 2. Figure 2a shows month by month variation of moisture content of coal. It can be observed that moisture of coal is minimum in June, i.e. peak of the summer. Subsequently coal becomes wet due to rains having maximum moisture in the month of August i.e. monsoon. After that, coal starts getting dried and shows lower moisture up to November. Since then, moisture content increases due to occasional rains, dew drops, colder ambient conditions and humidity level being quite close to dew point up to 1st January. From January to June i.e. spring and pre-summer, moisture content decreased due to dry ambient conditions in Singrauli coalfield area. Overall moisture of coal varied from 9.6% in June to 14.5% in August (Fig. 2a). Hence, at any point of time the stockpile coal has minimum 9.6% moisture content possibly indicating the coal to be hygroscopic in nature. That is typical for a relatively low rank bituminous coal where the oxidation rate is affected by relative humidity and moisture of coal (Bhatt and Agarwal 1996).
Figure 2b shows month by month variation of volatile matter content of the stockpile coal. Significant difference can be observed between the morning and evening samples for the month of December. In case of both the samples, however, volatile matter content increases within a month of storage. Initially the stockpile coal (1st November 2015) had 23.9% VM, that increased within a month to 25.3%. Initial increase in volatile content results due to adsorption of oxygen from the atmosphere. Subsequent erratic, but gradual decrease till 1st June 2016 to the level of 22% is possibly due to oxidation at low temperature with release of combustion products (Banerjee et al. 2000; Leonard 1977; Kus and Kennan 2017; Jose et al. 1996) and natural decomposition of various hydrocarbons as described in Sect. 3.1. During June and subsequent monsoon months, volatile matter consistently increases possibly because of natural decomposition of fixed carbon and producing thereof volatile matter by oxidation process, weather (alternatively intense heat and intense rain) being conducive to that (Leonard 1977; Kus and Kennan 2017; Fredricks et al. 1983). As a result seasonal variation of volatile matter content in coal (Fig. 2b), do not appear to be that significant, overall yearly value being 23.7 ± 1.6%.
Figure 2c shows month by month variation in fixed carbon content of the stockpiled coal. There is practically no difference in the values between morning and evening samples. Fixed carbon of coal from its highest value of 38.2% get decreased during storage of eleven calendar months to its lowest value of 17.2%, a drop by more than a half. Rate of decrease is however not uniform. It is maximum in the initial months from November to February i.e. winter, from 38.2% to 28.9%, a drop by 9.3%. During winter (months of December to February), with temperate climate, decline is small, from 28.9% to 28.3%, i.e. 0.6%. With the onset of summer, rate of decrease is more again, from 28.3% to 25.9%, i.e. 3.4%. Another sharp drop in fixed carbon content is observed in the months of June and July, from 25.9% to 19.4%, i.e. 6.5%. This decrease can be correlated with the increase in the content of volatiles in monsoon months. The results suggest that fixed carbon get converted to volatile matter by oxidation in humid conditions (Fig. 2c). Fixed carbon content decline appears to be due to the heat release on wetting because of the stockpiled coal exposed to alternate sunshine + dry climate and rain + humid weather. The heat release during of wetting can range between 85 and 105 J/g that raises the coal temperature by 25–30 °C (Berkowitz 1979) and consequently increases the oxidation rate.
Figure 2d shows month by month variation of ash content of coal practically with no change in the morning and evening samples. It can be observed that ash percentage of coal gets increased from 27.2% to 46.6% during the period of storage. Overall increase in ash is 19.4% with average monthly increase of 1.76%. Rise in ash content is due to the fact that, during storage carbonaceous materials becomes oxidized/decomposed and overall weight of coal get reduced. In case of a high rank Indian coal, ash content increased by about 8% during a seven month open air stockpiling (Banerjee et al. 2000).
3.3 Effect of weathering on fuel ratio, combustibles and non-combustibles ratio of coal
Figure 3 shows the month by month variation of fuel ratio (FC/VM) and total combustibles (FC + VM) of the stockpile coal. Since volatile matters do not show significant change through the time period with some increase in its content at the end while fixed carbon continuously decreases, total combustibles shows a continuously declining trend. Decrease in total combustibles suggest that stockpile coal kept on losing its calorific value during storage. Because of the relatively small change in volatile matter content compared to large changes in fixed carbon, extent of decrease in fuel ratio might appear to be small in some of the months. Hence, utilization of weathered coal, apart from massive heat loss, through unsteady combustion may result in operational difficulty in boiler.
3.4 Effect of weathering on GCV
It has already been reported that carbon and hydrogen content of the stockpile coal, both significantly contributing to its heat value, declined through the one year period due to the heat release on wetting because of the stockpiled coal exposed to alternate rain + humid weather and sunshine + dry climate. In addition, rise in sulfur content through highly exothermic reaction further contributed to the process of spontaneous heating. Both appear to have contributed to decay or disintegration of coal matter through incomplete or complete combustion, though no fire has been reported from the coal stockpile. As a result, GCV of the relatively low rank coal in stockpile decreased on month to month basis from 4578 kcal/kg for the month of November to 2751 kcal/kg for the month of October next year, a drop by 1827 kcal/kg equivalent to approximately six commercial grades in vogue in India. Seasonal decline of GCV of the coal in open air stockpile is observed for the whole 1 year (Fig. 4). The GCV declining trend observed in case of this investigation is in line with a previous study carried out for a high rank North Karanpura coal also from India (Banerjee et al. 2000). The said study reported a GCV drop of about 600 kcal/kg in just 5 months covering summer and rainy seasons.
Figure 5 shows the changes in percentage variation of combustible (FC + VM), carbon, gross calorific value and relative ash percentage with consecutive six calendar months from November 2015 to September 2016, i.e. winter and pre-monsoon, next year. From Fig. 5, it can be inferred that with increase in storage duration, combustibles (FC + VM) get reduced from 100% (November 2015) to 70% (September 2016), a decline by marginal 30%. However, Steady decline in reduction of combustibles is observed for the first three initial months, November–February 2016 (winter). During pre-monsoon period (months of March–June), nominal decrease is observed, thereafter it drops to a low of 65%. This variation in decline could be attributed to change in volatile matter and fixed carbon content which results in a steady drop in GCV by 600 kcal/kg. Similarly, carbon percentage reduces monotonously throughout the first 6 months of storage which also affects its GCV due to weathering. This decline in carbon content by 35% is a major cause of concern for trading thermal coal, a primary source of energy for its use in power utilities. As reported in previous section, carbon content, a significant contributor to heat value declined through the calendar period due to exposure of coal to alternate rain + humid weather and sunshine + dry climate. On the other hand, relative ash percentage increases from 1.0% (November 2015) to 1.7% (September 2016). Overall increase in relative ash percentage could be ascribed to aerial oxidation/decomposition of carbonaceous materials present in coal which significantly reduces the weight of coal. Relative ash percentage is calculated from the formula as shown in Eq. (9):
3.5 Effect of weathering on combustion behavior
3.5.1 TGA–DTG behavior of coal for the eleven calendar months
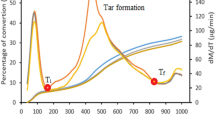
Figure 6 shows the result obtained from combustion studies carried out in TGA unit from room temperature to 650 °C in the presence of oxygen gas with a heating rate of 10 °C per minute. TGA results show that, with increase in temperature, initially 7%–15% decrease in weight loss occurs below 150 °C due to removal of moisture content from the coal samples. In the temperature range of 150–300 °C, 1%–5% weight gain is observed due to adsorption of oxygen gas at the porous structure of coal formed after moisture removal. After 300 °C, sharp decrease in weight loss is observed signifying the initiation of combustion process. Various burning profile temperatures like \(T_{i}\), \(T_{p}\) and \(T_{f}\) were identified from DTG curve shown in Fig. 7. Overall, \(T_{i}\) varied between 297 and 331 °C across different weathered coal samples from November to October 2016 month of next year which are summarized in Table 1. Within the temperature range 392–418 °C, sharp decrease in weight occurs, signifying rapid combustion of coal due to burning of fixed carbon content.
Corresponding (\(T_{p}\)) and (\(DTG_{ \hbox{max} }\)) are reported in Table 1. In temperature range between 466 and 485 °C, weight loss becomes negligible, shows constant weight, signifying completion of combustion process, with left out ash residue. From Table 1, it can be observed that \(T_{i}\), \(T_{p}\), \(T_{f}\) increases with weathering months of stockpile coal. Increase in the characteristics temperatures (\(T_{i}\), \(T_{p}\), \(T_{f}\)) as summarized in Table 1 were due to decrease in combustible material in coal. Decrease in combustible materials and increase in ash content from 27.2% to 46.6%, led to the decrease in \(DTG_{ \hbox{max} }\). An important observations related to changes in combustion characteristics temperatures (\(T_{i}\), \(T_{p}\), \(T_{f}\)) are their ranges due to combustion. As all these parameters are correlated with the residence time in furnace, any deviations in any of the parameters causes unburned carbon generation in furnace surface. For these purpose typically, \(T_{f}\) is considered as a most important parameter for the burning residence time of coal which affects its performance. However, overall variations in ignition temperature (\(T_{i}\)) is about 313 ± 9 °C for all the coal samples. This variation of temperatures are due to variation in volatile matter content as it is well known for initial ignition of coal which varies with increase in storage period as mentioned in section above. Similar variation in peak temperature and burnout temperature can be observed at 404 ± 13 °C and 475 ± 10 °C respectively. These variations are due to distribution of ash and fixed carbon content, as presence of carbonaceous material that lead to superior most combustion performance. Table 1, further describes the corresponding \(DTG_{\hbox{max} }\), signifying the relative presence of porous structures and volatile matter content in coal. Higher value of DTGmax signifies smooth burning of coal by release of hydrocarbons as can be seen from Fig. 7. Overall, it is recommended that stockpile coal should not be kept in weathered condition for not more than five to six calendar months as increase in storage period hamper the burning performance in boilers and decreases its efficiency (Fig. 8).
3.5.2 Effect of weathering on combustion performance indices
The combustion and comprehensive indices of the naturally weathered coal are represented in Table 2. With increase in storage duration from the month of November 2015 to October 2016, \(D_{i}\) shows significant drop from 16.2 to 5.52 wt%/min3 which can be seen in Table 2. This is due to decrease in volatile matter content from 23.93% to 23.44% with partial oxidation, resulting in greater volatility and adsorption of oxygen gas from atmosphere during storage period. Coal with high volatile matter content contributes faster de-volatilization process that leads to lower particle ignition temperature \((T_{i} )\) 297 °C for the month of December 2015 as mentioned in Table 1, corresponding to maximum ignition index (\(D_{i}\)) i.e. 15.6 wt%/min3. However, ignition index (Di), during the storage of winter i.e. the month of November, December and January is generally higher i.e. 16.2, 15.6 and 15.6 wt%/min3 compare to the month of February, March, April and May (winter and pre-monsoon) which shows a decreasing trend in Di i.e. 7.72, 7.92, 5.87 and 6.64 wt%/min3. Similar trend is observed for burnout index (\(D_{f}\)) as shown in Table 2. It is therefore observed that the results of burnout performance matched are substantially in accordance with the results of ignition performance the reason being \(D_{f}\) represents the combustion performance of coal from peak temperature (\(T_{p}\)) region to burnout temperature (\(T_{f}\)) region. Coal samples have lower \(T_{p}\) and \(T_{f}\) value 391 °C, 392 °C, 397 °C and 465 °C, 468 °C, 470 °C for the month of November, December and January (winter period) with maximum \(D_{f}\) value i.e. 4.62, 4.46 and 4.22 wt%/min4. Therefore, coal having higher \(D_{f}\) value signifies better combustion process. However, different values are not similar; as because \(D_{i}\) and \(D_{f}\) depends on other factors too. Similar case is observed with combustion performance index (S) which decrease from 4.15 (November 2015) to 3.2 (October 2016) wt%2/min2 C3. This is due to increase in \(T_{i}\) from 296 to 331 °C as shown in Table 1. In accordance with the results of ignition index of coal having higher \(DTG_{ \hbox{max} }\) i.e. 16.14 wt%/min possess better combustion performance due to rapid de-volatilization process and affects the carbon and oxygen reaction which enhance the combustion performance. On the other hand, \(H_{f}\) value increases with increase in storage duration of stockpile coal, which can be observed in Table 3. Maximum \(H_{f}\) value 0.82 is observed for the longest duration during the month of September 2016, having lower \(DTG_{ \hbox{max} }\) 4.72 wt%/min and higher value of \(T_{p}\) i.e. 416 °C, which is undesirable for a coal samples for proper combustion. Thus, lower value of \(H_{f}\) shows good combustion intensity rate. Therefore, analyzing the indices parameters, it is similarly suggested stockpile coal should be restricted to not more than five to six calendar months on an average storage period.
3.5.3 Analysis of Arrhenius parameters
Table 3 represent the estimated kinetic data on combustion of coals which were obtained using Coats–Redfern method. It can be seen that the present results of the activation energy ranges from 21.15 to 53.98 kJ/mol for coal samples for the month from November 2015 to October 2016. Activation energies during the winter period (month of November 2015, December 2015 and April 2016) are quiet low than other months, which are around 20 to 30 kJ/mol signifying better combustion characteristics as ash content is low and volatile matter doesn’t vary within this range. However, samples during longer duration of storage shows higher activation energies due to absence of higher amount of carbonaceous material owing to un-oxidized state during storage period. Table 3 shows that activation energy of coal for the month of February, March 2016 are in similar range with coal storage during pre-monsoon and monsoon (month of May, June and July 2016). Activation energies are less in case of coal storage during the month of November 2015, December 2015 and April 2016 (i.e. winter and early summer) signifying coals are easier to burn as activation energy supposed to be independent of coal type and its properties. By and large from activation data, stockpiled sample for the first six calendar months is recommended for its use in utilities as activation energy lies in the permissible range of 30 to 35 kJ/mol (Wagner 2008).
4 Conclusions
In this study, impact of weathering on various properties of coal during long term storage was investigated. Experimental studies shows that carbon and hydrogen content of coal decreases from 46.6% to 28.6% and from 3.3% to 2.9%, respectively during the 330 days of storage period of coal. Decrease in carbon and hydrogen percentage results in decrease in gross calorific value of coal from 4578 to 2751 kcal/kg, fixed carbon content from 35.6% to 19.9%, with an increase in ash percentage from 29.2% to 46.6%. Due to removal of hydrogen % and increase in ash %, ignition temperature of coal increased from 293 to 331 °C and activation energy from 22 to 54 kJ/mol. Increase in activation energy indicates deterioration in coal burning behavior with long duration of storage. Further, Tp and Tf also increased from 391 to 409 °C and 465 to 478 °C. This increase in burning temperatures are due to loss of carbonaceous material from stockpile coal during weathering. Detailed analysis of various combustion indices (Di, Df, Sf and Hf) shows that due to weathering, combustion of coal becomes difficult. Further, ultimate analysis results shows that sulfur content increased from 0.33% to 1.08% during storage. Rise in sulfur content contributes to spontaneous heating of stockpiled coal decreasing the combustion performance of the weathered coal, which results in operational difficulty in the boilers. Overall proximate, ultimate and gross calorific value results suggest that stockpiling of coal should be restricted to not more than a month whereas, sulfur content variation suggests that stockpiling can be done for a period of not more than six calendar months.
References
Banerjee D, Hirani M, Sanyal SK (2000) Coal quality deterioration in a coal stack of a power station. Appl Energy 66:267–275
Berkowitz N (1979) An introduction to coal technology. Academic Press Inc, New York
Bhatt S, Agarwal PK (1996) The effect of moisture condensation on the spontaneous combustibility of coal. Fuel 75:1523–1532
Bhattacharaya S, Maitra AK (2007) Impact of coal beneficiation on rail transport in India. Coal Prep 27:149–166
Cimadevilla JLG, Alvarez R, Pis JJ (2005) Effect of coal weathering on technological properties of cokes produced at different scales. Fuel Process Technol 86:809–830
Crelling JC, Schrader RH, Benedict LG (1979) Effects of weathered coal on coking properties and coke quality. Fuel 58:542–546
Ebrahimi-Kahrizsangi R, Abbasi MH (2008) Evaluation of reliability of Coats–Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferrous Met Soc China 18:217–221
Fredricks PM, Warbrooke P, Wilson MA (1983) Chemical changes during the natural oxidation of high volatile bituminous coal. Org Geochem 5:89–97
Jose P, Puente G, Fuente E, Moran A, Rubiera F (1996) A study of self-heating of fresh and oxidized coal by differential thermal analysis. Thermochim Acta 273:93–101
Ken BS, Aich S, Saxena VK, Nandi BK (2018) Combustion behavior of KOH desulphurized coals assessed by TGADTG. Energy Sources A Recovery Util Environ Effects 40:2458–2466
Kříbek B, Sýkorová I, Veselovský F, Laufek F, Malec J, Knésl I, Majer V (2017) Trace element geochemistry of self-burning and weathering of a mineralized coal waste dump: the Novátor mine, Czech Republic. Int J Coal Geol 173:158–175
Kus J, Kennan MM (2017) Coal weathering and laboratory (artificial) coal oxidation. Coal Geol 171:12–36
Leonard JW (1977) Coal preparation, 4th edn. AIME, New York
Smędowski Ł, Piechaczek M (2016) Impact of weathering on coal properties and evolution of coke quality described by optical and mechanical parameters. Int J Coal Geol 168:119–130
Tamamura S, Ueno A, Aramaki N, Matsumoto H, Uchida K, Igarashi T, Kaneko K (2015) Effects of oxidative weathering on the composition of organic matter in coal and sedimentary rock. Org Geochem 81:8–19
Wagner NJ (2008) The characterization of weathered discard coals and their behaviour during combustion. Fuel 87:1687–1697
Xia W, Yang J (2014) Changes in surface properties of anthracite coal before and after inside/outside weathering processes. Appl Surf Sci 313:320–324
Zhang Y, Guo Y, Cheng F, Yan K, Cao Y (2015) Investigation of combustion characteristics and kinetics of coal gangue with different feedstock properties by thermo gravimetric analysis. Thermochim Acta 614:137–148
Acknowledgements
The Investigation was sponsored by Vindhyachal Power Station of National Thermal Power Corporation Limited Vide Work Order No: 5241-291-6628 dated 16 February 2016. Any opinions, findings and conclusions expressed in this paper are those of the authors and do not necessarily reflect the views National Thermal Power Corporation Limited, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aich, S., Nandi, B.K. & Bhattacharya, S. Effect of weathering on physico-chemical properties and combustion behavior of an Indian thermal coal. Int J Coal Sci Technol 6, 51–62 (2019). https://doi.org/10.1007/s40789-018-0235-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-018-0235-0