Abstract
The influence of main characteristics upon conversion directions of the lignite organic part during its oxidation desulphurization was studied. The optimum temperature values, the ratio oxidant : raw material, and time of coal stay in the reaction zone, which provide the maximum degree of sulphur conversion and hydrogen sulphide content in desulphurization gases, were calculated. The process implemented under these conditions will decrease environment pollution by sulphur dioxide during further lignite burning at least to 55 %–60 % and utilize sulphur in coal in the form of desulphurization gases with hydrogen sulphide content of 7 %. Such gases can be reprocessed by the known methods of obtaining sulphur. The effect of the above three factors on the depth and character of the coal organic matter transformation was studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well-known that sulphur oxide in coal is the biggest environment pollutant, as far as the coal is the main raw material for thermal power plants and it comprises a lot of sulphur compounds (Statistical Review of World Energy 2013; Sulphur dioxide SO2 emissions (APE 001)—Assessment 2014; Pyshyev et al. 2012).
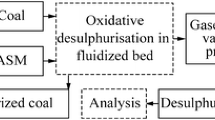
It was showed the principal possibility of reducing SO2 emissions while using the lignite desulphurization by oxidative method (Gunka and Pyshyev 2014). The process lies in processing lignite with a steam–air mixture or only with air that results in the conversion of 55 %–65 % of general sulphur into H2S. The conditions, i.e. oxidant linear rate and coal grain size, provide reaction proceeding between oxidant and sulphur in coal in kinetic area. Besides, it has been determined that to increase the content of H2S in desulphurization gases, it is reasonable to use the steam–air mixture instead of air.
In addition to the aforementioned factors, there are other ones which substantially influence coal desulphurization, in particular temperature, oxidant consumption and reaction period (Pyshyev et al. 2004, 2007, 2012). Taking into consideration all mentioned above, the further goal of our research was to determine the influence of the aforementioned factors upon the process of oxidative lignite desulphurization.
2 Experimental
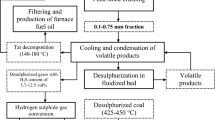
The sample of lignite for investigation was taken from the Morozivs’ke coal-field of Dnieper lignite basin. The technical analysis was carried out and different sulphur forms such as organic sulphur (\(S_{\text o}^{\text d}\)), pyritic sulphur (\(S_{\text{p}}^{\text{d}}\)) and sulphate sulphur (\(S_{{{\text{SO}}_{ 4} }}^{\text{d}}\)) were determined. The fraction of 0.1–0.25 mm was used for researches because it is the optimum size for coal burning at thermal power. The results are given in Table 1.
The investigations were conducted on the laboratory plant based on fludized bed reactor that functioned in the mode close to isothermal. The scheme of the laboratory plant and its detailed description were presented (Pyshyev and Hayvanovych 1996).
The desulphurizative gases were analyzed by means of gas-adsorptive chromatography, using a chromatograph “LHM” (N 479). The analyzed gas (10.0 mL) was introduced by a metering device into the flow of gas carrier (helium). Gas was fed into the first column (diameter of 2.0 mm and length of 3.0 m) filled with a non-polar sorbent Polysorb-1 and then, into the second column (diameter of 2.0 mm and length of 4.5 m) filled with a polar adsorbent—zeolite of CaX type (Pyshyev et al. 2007, 2012).
To characterize the relative rate of sulphuric compounds conversion, we used the term “total sulphur conversion (TSC)”. This value indicated the amount of sulphur converted into gaseous sulphur-containing products that will not be in the atmosphere at further burning of desulphurized coal (the level of environmental pollution decreases). It is calculated in accordance with the Formula (1), %:
where \(S_{{{\text{t}}\; 0}}^{\text{a}}\) is the content of total sulphur in the initial coal relative to the analytical sample, mass%; \(S_{\text{t}}^{\text{a}}\) is the content of total sulphur in the desulphurized coal relative to the analytical sample, mass%; and \(x_{\text{C}}\) is the yield of desulphurized coal, mass%.
The sulphur content in the desulphurized coal depends on the ratio between the conversion rates of the coal and pyritic sulphur in it. Hence, the removal degree of pyritic sulphur (RDPS) was calculated in accordance with the Formula (2) and indicated the ratio between the rate of FeS2 conversion followed by the production of gaseous products and the rate of organic matter reaction, i.e. process selectivity:
where \(S_{{{\text{p}}\; 0}}^{\text{d}}\) is the content of pyritic sulphur relative to the dry sample, mass%; \(S_{\text{p}}^{\text{d}}\) is the content of pyritic sulphur in the desulphurized coal relative to the dry sample, mass%.
To characterize the oxidant consumption, the term “repetition factor of oxidant flow rate” (OFR) was used. OFR was calculated as the ratio between the volumetric flow air–steam mixture (m3/h) and coal mass (kg).
While describing conversions which occurred with the coal organic matter (COM), we assumed that conversions proceed in three directions, i.e. thermal destruction (decomposition tar is formed); gasification with the formation of combustible gases (hydrogen, hydrocarbons, CO); burning (CO2 is obtained). The amount of COM necessary for production of all aforementioned products is characterized by the term “coal organic matter conversion” (COMC). To characterize the ratio between the amount of COM from which combustible products are formed (tar, hydrocarbon gases, carbon(II) oxide) and the amount of burned COM (for CO2 formation), we used the term “efficiency factor of coal organic matter conversion” (EFCOMC).
where \(x_{{{\text{CH}}_{ 4} }} ,\;x_{{{\text{C}}_{ 2} - {\text{C}}_{ 3} }} ,\;x_{\text{CO}} ,\;x_{{{\text{CO}}_{ 2} }}\) are the concentrations of corresponding components in desulphurization gases, vol%; \(M_{{{\text{CH}}_{ 4} }} ,\;M_{{{\text{C}}_{ 2} - {\text{C}}_{ 3} }} ,\;M_{\text{CO}} ,\;M_{{{\text{CO}}_{ 2} }}\) are the molecular masses of methane, C2–C3 are the mixture and carbon, respectively; V dg is the volume of desulphurization gases, m3; m C is the initial coal mass, kg; x t is the tar yield, mass%.
The modular degree of oxygen conversion was calculated by the following formula:

where \(x^{\prime }_{{{\text{o}}_{ 2} }}\) and \(x^{\prime }_{{{\text{N}}_{ 2} }}\) are the content of corresponding components in desulphurization gases, vol%; 78.08 and 20.95 are the concentration of nitrogen and oxygen in the resulted air, vol%.
3 Results and discussion
3.1 Temperature influence
Taking into account the fact that the lignite organic matter is thermally unstable, has the high reaction capability, contains many functional oxygen containing groups, and is not caked, the temperature impact was studied at relatively low and high temperature ranges (200–500 °C). Experimental results at different temperatures are given in Table 2 and in Figs. 1 and 2.
Temperature rise results in the increase of COMC (Fig. 1). These facts correlate with the content rise of CO2 and CO in desulphurization gases (Table 2). Temperature influences the directions of OMC insignificantly, i.e. at its changing EFCOMC is almost constant (Fig. 1).
If the temperature reaches 425 °C, RDPS and TSC (Fig. 2) as well as H2S in desulphurization gases rise (Table 2). Removal degrees of sulphur characterize with the ratio between reaction rates of sulphur conversion and COM. The greater is the ratio, the less is the sulphur content in obtained coal and appropriately the sulphur removal degree is the higher. That is why we can state that if temperature exceeds 425–450 °C, the conversion rates of sulphur compounds and COM become equal.
It is understood that in case of temperature rise, the increase of sulphur conversion intensity and COM will facilitate the decrease of non-converted quantities oxygen in desulphurization gases and К rise (Table 2).
Taking into consideration the aforementioned, the optimum temperatures should be considered close to 425–450 °C.
3.2 Influence of the ratio of oxidant : raw material
Reaction rate of gaseous-phase reagents with solid bodies and conversion degrees of final substances are substantially influenced by the ratio between the number of reagents. To characterize this ratio, we used the notion of OFR.
Concerning the process under research, we can state that it should be conducted with the least oxidant losses, which would provide sufficiently high sulphur removal and conversion degrees, as far as the ratio rise of oxidant consumption (m3/h) to coal mass (kg) will result in increasing volume of desulphurization gases and appropriately decreasing the content of substances of sulphur conversion in them.
The results obtained are presented in Table 3 and Figs. 3 and 4.
In case of the increase of oxidant, that is being given per unit of raw material, insignificant rise of COMC (Fig. 3) occurs, and the content of oxygen-containing gaseous products (CO2 and CO) decreases twice if OFR rises, and appropriately, and the volume of desulphurization gases increases eight times (from 0.6 to 4.8 m3/(h kg), Table 3). During the process, COMC can decrease as a result of gasification reactions, thermal decomposition and burning, and can increase resulting from adhesion of oxygen and water steam by the organic part (synthesis reaction). Taking into consideration the insignificant rise of COMC and absolute quantity of CO and CO2, it can be stated that OFR rise to a great extent contributes to destruction reactions than to the synthesis of COM. It should be noticed that to a considerable extent, the OFR rise intensifies the reactions of COM burning more than the reactions of its destruction and gasification, as far as EFCOMC decreases (Fig. 3).
The process implementation without oxidant feed (OFR = 0) causes sufficiently high degree of sulphur conversion. It is confirmed by the proposal that the conversion reactions of sulphurous compounds of lignite take place mainly due to the participation of the reactive part of COM in them. If small quantity of oxidant (OFR = 0.6 m3/(h kg)) is fed in the reaction zone, the process intensification of sulphurous compound conversion occurs (Fig. 4). It indicates that air oxygen takes insignificant part in the reactions of pyritic sulphur conversion. The increase of OFR from 0.6 to 4.8 m3/(h kg) leads to insignificant decrease of sulphur removal and conversion degrees, since OFR rise intensifies gasification and thermal destruction of organic matrix, that causes the reduction of the number of the reactive parts of COM.
All aforementioned allows to recommend conducting the process of lignite desulphurization with minimum admissible values of OFR (close to 0.6 m3/(h kg)), which provide maximally possible RDPS, TSC and the content of H2S in desulphurization gases and these are sufficient for keeping up the process temperature due to burning out the part of COM.
3.3 The influence of process time
The reaction intensity in the desulphurization system, that consists of solid body and gas, substantially depends upon the ratio of reagents and the period of their contact. That is why it is so important to define which should be the optimum time of the process with the minimum oxidant consumption [close to 0.6 m3/(h kg)]. The obtained results are presented in Table 4 and Figs. 5 and 6.
The obtained data make it possible to state that the increase of process time causes the deepening of the burning processes, gasification and decomposition of COM, that results the rise of COMC (Fig. 5). Moreover, the time rise primarily intensifies the reactions of burning the organic part (EFCOMC decreases).
Intensity rise of coal matrix burning with time increase of raw material stay in the reactor is confirmed by the insignificant decrease of CO2 content in desulphurization gases, though the volume of gases increases directly proportional to the process time increase (Table 4).
If the process time is over 10–15 min, the COM conversion reactions take place less intensively, that is confirmed by the decrease of К (Table 4).
According to the data of Figs. 1 and 2 the sulphur conversion takes place at the beginning of the process, i.e. the maximum degrees of sulphur removal and conversion are within 5–10 min. The further increase of the process time does not lead to significant increase of RDPS and TSC (Fig. 6).
Thus, the optimum process time should be considered 5–10 min, as far as its further increase does not lead to significant rise of RDPS and TSC, but causes the reduction of H2S quantity in desulphurization gases.
4 Conclusions
In case of lignite desulphurization, RDPS and TSC depend substantially on temperature, whose optimum values should be considered 425–450 °C. The process can be carried out with the minimum quantities of oxidant (0.6 m3/h air/1 kg of coal) during 5–10 min. Relatively high degrees of lignite sulphur are reached while heating raw material without air. All these data are useful to confirm the assumption suggested that in the course of lignite desulphurization, the main sulphur conversions take place as a result of pyritic sulphur reaction with high reaction organic mass and/or the products of its destruction and gasification (Pyshyev et al. 2012). Only insignificant number of reactions of sulphurous compound conversion is carried out with oxidant part.
It is known according to Gunka and Pyshyev (2014) that the use of water steam in oxidant (in the mixture with air) practically does not influence the intensity of sulphurous compound conversion, but makes it possible to increase the content of sulphur convertion products in desulphurization gases. In the researches presented in the paper, only air was used as an oxidant. Even in this case, it is possible to obtain desulphurization gases with the content of hydrogen sulphide equal to 7 vol%, that will make it possible to reprocess them for obtaining element sulphur by the known methods (Grunvald 1992; Javorskyi 2010).
The general sulphur conversion degrees are equal to the sulphur quantity that passes to the gas phase, and in case of further coal burning, it does not get into the atmosphere. That is why the results obtained allow stating that with the use of the process in thermal power, we can decrease environment pollution by sulphur dioxide at least to 55 %–60 %.
References
Grunvald V (1992) Technology sulphur gas, Khimiya, Moskwa
Gunka V, Pyshyev S (2014) Lignite oxidative desulphurization. Notice 1. process condition selection. Int J Coal Sci Technol 1(1):62–69
Javorskyi VT (2010) The technology of sulphur and sulphuric acid. Lviv Polytechnic, Lviv
Pyshyev S, Hayvanovych V (1996) Laboratory plant of coal desulphurization via oxidation method. Sci Present Lviv Polytech State Univ 298:96–98
Pyshyev S, Gayvanovych V, Pattek-Janczyk A, Stanek J (2004) Oxidative desulphurization of sulphur-rich coal. Fuel 83:1117–1122
Pyshyev S, Shevchuk K, Cmielarz L, Kustrovski P, Pattek-Janczyk A (2007) Effect of water-vapor content on the oxidative desulfurization of sulphur-rich coal. Energy Fuel 21:216–221
Pyshyev S, Gunka V, Prysiazhnyi Y, Shevchuk K, Pattek-Janczyk A (2012) Study of oxidative desulphurization process of coal with different metamorphism degrees. J Fuel Chem Technol 40(2):129–137
Statistical Review of World Energy (2013) http://www.bp.com/content/dam/bp/pdf/statistical-review/statistical_review_of_world_energy_2013.pdf
Sulphur dioxide SO2 emissions (APE 001)—Assessment (2014) http://www.eea.europa.eu/data-and-maps/indicators/eea-32-sulphur-dioxide-so2-emissions-1/assessment-3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gunka, V., Pyshyev, S. Lignite oxidative desulphurization. Notice 2: effects of process parameters. Int J Coal Sci Technol 2, 196–201 (2015). https://doi.org/10.1007/s40789-015-0056-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-015-0056-3