Abstract
Purpose of Review
A major cause of visual disorders is dysfunction and/or loss of the light-sensitive cells of the retina, the photoreceptors. To develop better treatments for patients, we need to understand how inherited retinal disease mutations result in the dysfunction of photoreceptors. New advances in the field of stem cell and gene editing research offer novel ways to model retinal dystrophies in vitro and present opportunities to translate basic biological insights into therapies.
This brief review will discuss some of the issues that should be taken into account when carrying out disease modelling and gene editing of retinal cells. We will discuss (i) the use of human induced pluripotent stem cells (iPSCs) for disease modelling and cell therapy; (ii) the importance of using isogenic iPSC lines as controls; (iii) CRISPR/Cas9 gene editing of iPSCs; and (iv) in vivo gene editing using AAV vectors.
Recent Findings
Ground-breaking advances in differentiation of iPSCs into retinal organoids and methods to derive mature light sensitive photoreceptors from iPSCs. Furthermore, single AAV systems for in vivo gene editing have been developed which makes retinal in vivo gene editing therapy a real prospect.
Summary
Genome editing is becoming a valuable tool for disease modelling and in vivo gene editing in the retina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited Retinal Degenerative Diseases
Vision impairment is a global health issue estimated to affect more than 285 million people worldwide [1]. Over 50% of all visual impairment cases in the developing world are the result of dysfunction and/or loss of photoreceptors, a specialized type of neuron that performs the essential first step in transforming light into vision [2]. Inherited retinal diseases such as retinitis pigmentosa (RP), Leber congenital amaurosis (LCA), Stargardt disease, as well as more complex and heterogeneous retinal diseases such as age-related macular degeneration (AMD), are among the most common types of retinal degeneration [3]. Inherited retinal diseases have been associated with mutations in more than 200 different genes (see http://www.sph.uth.tmc.edu/Retnet) [4, 5]. As a result, the onset of hereditary disease and the speed of progression can be highly variable. This contrasts with age-related macular degeneration (AMD) which specifically affects older adults and where dysfunction of photoreceptors is thought to be caused by cellular senescence of retinal pigment epithelium cells (RPE), a photoreceptor support cell [6,7,8].
Compared with many other parts of the nervous system, the eye represents a highly accessible and (at least partially) immune-privileged system [9,10,11]. It is therefore unsurprising that it has become a focus of significant translational research efforts. Common to all vertebrate retinas is a highly stratified structure, composed of three layers of cells connected by two synaptic layers. The outer retina is composed of photosensitive cone and rod photoreceptor cells, which form the outer nuclear layer (ONL), connecting to interneurons including bipolar, amacrine and horizontal cells in the inner nuclear layer (INL) (Fig. 1). Notably, most forms of inherited retinal diseases affect photoreceptors or their support cells (e.g. RPE), resulting in photoreceptor death, but the inner retina (i.e. bipolar, amacrine, horizontal and ganglion cells) remains largely unaffected [3, 12, 13]. This makes the retina an attractive recipient for novel therapeutic approaches including gene and cell therapy and the use of implant devices.
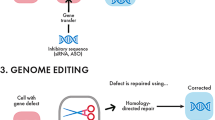
Schematic diagram depicting how CRISPR/Cas9 gene editing can be harnessed for in vitro disease modelling and in vivo gene editing. CRISPR/Cas9 gene editing panel: Cas9 nuclease in complex with a gRNA can generate specific double-stranded breaks (DSB) in the host DNA. Cell’s DNA repair mechanism will repair the DSB by either error-prone NHEJ or error-free HDR. NHEJ-mediated gene editing will most likely result in the introduction of insertions and deletions (indel, red lines) that will lead to premature STOP codon formation. HDR-mediated gene editing, in the presence of a homologous DNA template (green lines), will introduce precise genomic changes in the host’s DNA. Most popular CRISPR/Cas9 gene editing approaches are an RNP approach, where Cas9 protein is complexed with gRNA for delivery, and a plasmid approach, where Cas9 cDNA, gRNAs and a reporter are usually overexpressed from one, two or more vectors. In vitro disease modelling panel: Shows how fibroblasts can be sampled from healthy and affected individuals and reprogrammed into human iPSCs. CRISPR/Cas9 gene editing can then be used to introduce disease-causing mutations of interest in healthy iPSCs or correct them in affected iPSCs to generate isogenic iPS cell line pairs. These can then be differentiated to photoreceptors for disease modelling purposes. In vivo gene editing: cDNA containing Cas9 gene editing tool can be packed into AAV for subretinal injection to target different populations of retinal cells. The retina is a layered structure composed of three layers of cells connected by two synaptic layers: the inner plexiform layer (IPL) and the outer plexiform layer (OPL). At the outer most region, cone and rod photoreceptor cells form the outer nuclear layer (ONL). The inner nuclear layer (INL) is composed of bipolar, amacrine, horizontal and Müller glia cells. Lastly, the inner most layer, the ganglion cell layer (GCL) is comprised of retinal ganglion cells and displaced amacrine cells. The optic fiber layer (OFL) contains retinal nerve fibers that exit the eye through the optic nerve
Gene and Cell Therapies
Archetypal gene therapy aims to provide a normal copy of the gene that is faulty by delivering it via a viral vector [14]. The most effective vectors for retinal transduction are those based on adeno-associated viruses (AAV). These vectors can mediate efficient long-term gene transfer to both photoreceptors and RPE. Recombinant AAV vectors can be constructed with a number of different capsids (i.e. serotypes) that can lead to transduction of different retinal cell types depending on route of administration. AAVs are relatively small viruses with a single-stranded DNA genome. They are non-pathogenic to humans, and depending on their method of delivery, they can penetrate deep into tissues providing very good levels of cellular transduction. In the retina, subretinal delivery is currently the route of administration of choice with serotypes such as AAV5 and AAV8 providing highly efficient photoreceptor transduction. While the capacity of AAV vectors is relatively small when compared to other viral vectors, with a maximum capacity of approximately 5 kb of insert DNA, it is large enough to accommodate most genes. In preclinical studies over the last 15 years, the utility of AAV vectors for gene supplementation for recessively inherited degenerations has been demonstrated in a number of animal models leading to long-term phenotypic rescue [15, 16].
The success in preclinical development has led to a number of phase I/II clinical trials to treat inherited retinal degenerative diseases using gene therapy [17,18,19,20,21,22,23]. In the first trials, patients carrying a defective RPE65 gene, which causes a form of LCA, received a subretinal injection of an AAV vector carrying a human RPE65 complementary DNA (cDNA) under the control of either a human RPE65 promoter [17] or a ubiquitously expressed actin promoter [20, 23]. In each case, subretinal delivery of an AAV vector in patients was shown to be safe. Additional findings included improvement of visual function, although these improvements varied between patients and were modest overall. More recent reports have further demonstrated efficacy but have also underlined the importance of achieving the correct levels of gene expression for robust and sustained rescue [24,25,26]. Despite the success of these trials, a gene supplementation approach has its limitations; in particular, it requires the presence of the affected cells and is most effective in the early stages of disease progression. Another limitation is the length of the cDNA that the AAV can package for delivery [27,28,29,30], which prevents replacement of very large genes, such as ATP-binding cassette subfamily a member 4 (ABCA4, ∼7.3 Kb), which causes Stargardt disease [31, 32] and centrosomal protein of 290 kDa (CEP290, ∼7.9 kb) which causes another form of LCA [33]. Moreover, it can only be used for single-gene defects involving a known gene.
Stem Cell-Derived Photoreceptors
Cell replacement therapy offers a therapeutic approach for patients with advanced disease where there has been extensive photoreceptor loss. It may also permit treatment of conditions with non-genetic or unknown cause. We, and others, have carried out a variety of studies in the area of photoreceptor derivation and transplantation. Ground-breaking work on murine embryonic stem cells (ESCs) by Sasai and colleagues [34•] showed that ESCs cultured in 3D suspension in the presence of matrices, such as Matrigel, spontaneously form self-organising embryoid bodies that support the morphological differentiation of retinal cells. This so-called 3D culture technique has since been shown to sustain the differentiation of murine photoreceptors up to equivalents of the first postnatal week [35]. We, and others, have shown that donor photoreceptor cells from early mouse postnatal retina as well as ESC-derived mouse photoreceptor precursors can be transplanted into the degenerative retinas of adult mice [35,36,37,38,39,40,41,42,43]. Following transplantation, if present in sufficient numbers, these cells are capable of restoring aspects of visual function [42, 44,45,46,47]. Several laboratories have also described the derivation of photoreceptors from human ESCs and iPSCs [48,49,50,51,52,53,54, 55•, 56••]. Canto-Soler and colleagues were the first to show human pluripotent stem cells (hPSCs)-derived retinal organoid containing photoreceptors that bear key photoreceptors structures and are responsive to light in vitro [56••]. Their protocol uses a 3D to 2D to 3D multi-stage cell culture differentiation process that takes advantage of the ability of hPSCs to spontaneously differentiate towards the ectoderm pathway under neural induction culture conditions. In this protocol, unlike previous neuro-differentiation approaches [57,58,59], typically costly SMAD inhibitors are avoided in the neural induction process. Instead, hPSCs are allowed to form embryoid bodies (EBs) in suspension, then allowed to grow in a monolayer until “horseshoe”-shaped neural retinal-like structures appear spontaneously. These can then be dissected manually and cultured in suspension for up to 27 weeks to form mature retinal organoids. This hPSCS retinal differentiation protocol has an initial 3D to 2D step as others have previously described [48, 52, 53]. Among the advantages of these protocols is the ability to control the starting number of cells and, therefore, reduced initial variability. However, issues may arise when the resulting EBs are then grown on a 2D monolayer to further develop, as the presumptive neural retina structures are dissected manually. This appears to be a key stage in variability between different laboratories using the same protocol, as the criteria used to define the presumptive neural retinal structures is subjective. Canto-Soler and colleagues found that photoreceptors start to express mature markers, such as Rhodopsin, S-Opsin and LM-opsin, by week 17 and that these photoreceptors demonstrate light sensitivity by week 27. Goureau and colleagues on the other hand, used a simpler 2D to 3D cell culture system, where human iPSCs cells are allowed to become confluent on a monolayer, prior to neural induction [55•]. They take advantage of the cells’ ability to self-form into neural retinal-like structures even within the 2D cell culture conditions; these could then be dissected and further differentiated in 3D suspension, going on to correctly express many markers of retinal commitment and even opsin expression. While this protocol demonstrates a simpler way to differentiate iPSCs to neural retina, it failed to support the morphological differentiation of photoreceptors, including the formation of outer segments (OS), as described by Canto-Soler and colleagues [56••]. However, while Canto-Soler’s protocol supported Opsin expression and light sensitivity of hPSC-derived photoreceptors, the transmission electron microscopy (TEM) data showed little evidence of mature OS formation. Recently, we have adapted some of the protocols described previously and have found that it is possible to generate short OS-like structures from hESC-derived retinal organoids (Gonzalez-Cordero et al., in preparation). A recent report by Takahashi and colleagues showed that transplantation of immature hPSC-derived photoreceptors into the subretinal space of nude rats allows for the subsequent formation and maturation of OS structures [60••]. This suggests that the hPSC-derived photoreceptors described to date may all have the capacity to form OS but lack sufficient instruction or support from the in vitro environment to be able to do so.
A major issue currently limiting the widespread utility of hPSC-derived cells is the variability in efficiency between protocols and even the reproducibility of the same protocol by different laboratories. There are many potential variables that may be introduced, including the cell line used and its passage number, the different batch numbers of commercially produced reagents used and the subjective choice of neural retina resembling structures. Robust methods of quantifying the purity and viability, as well as the developmental stage, of photoreceptors will be important. As a step towards this, we, together with Sowden and colleagues, have used cluster of differentiation (CD) cell surface markers to quantify and purify stage-specific photoreceptors in mouse ES-derived photoreceptors and mouse and human retinal samples [40, 61]. Among these, CD73 allows for the enrichment of rod precursor cells [38, 40, 61]. Furthermore, pilot experiments using human retinal samples indicated that a similar CD panel could be used to assess and quantify the maturation stage of hPSC-derived photoreceptors, especially since human iPSC-derived photoreceptors and adult human retina are reported to express CD73 [55•, 61]. This approach, if successful in conjunction with human retinal organoids, may be useful for cell therapy purposes, as it circumvents the use of reporter markers in transgenic hPSC lines that cannot be used clinically. A CD panel could also be a good way to characterise and quantify the extent of retinal differentiation in individual batches, since previous studies have demonstrated that different hPSCs differentiate along the retinal lineage with varying efficiencies and with the resulting retinal cells almost always co-existing alongside non-retinal cells [48, 52, 54, 62,63,64]. An additional point to note is the need to develop robust criteria for defining what constitutes commitment of the stem cell to differentiate into a given cell type. At present, the field relies heavily on the presence/absence of various photoreceptor-specific proteins, typically assessed by immunocytochemistry. We have already shown that such methods of assessment can lead to erroneous conclusions about how well a cell has adopted a given fate [43] and therefore it will be important to assess the derived cells with greater thoroughness, using other assays such as RNAseq, morphology and functionality.
A challenge when differentiating human ES or iPSCs to retinal neurons using 3D culture methods is that, like mouse ES retinal differentiation, human ESC/iPSC retinal differentiation follows a time course similar to that of normal development, which for the human organoids is many months (Gonzalez-Cordero et al., in preparation); this leads to a large proportion of differentiated organoids becoming necrotic. This is a major hurdle, since photoreceptor OSs form late in development and need long periods of cell culture to form (Gonzalez-Cordero et al., in preparation). Recent findings from related ESC disciplines suggests that upgrading from the classic cell culture plate differentiation approach to the use of bioreactors can improve differentiation and viability, providing cells with improved aeration and distribution of nutrients, as well as apparently encouraging formation of 3D structures [65,66,67,68]. Preliminary work in our group suggests that bioreactor technology may represent a stepping stone to upscaling the cell culture process for therapeutic applications, together with the ability to improve the survival and abundance of mature photoreceptors (Ovando-Roche et al., in preparation).
Regardless of these challenges, however, it is clearly possible to generate functional photoreceptors from hPSCs. This raises the question of how best to use these advances to model retinal disease in vitro.
Using Human Pluripotent Stem Cells as Retinal Disease Modelling Tools
The inability of animal models to reflect human disorders accurately may have contributed to disappointing outcomes in many clinical trials of drugs and therapies. The revolution in somatic cell reprogramming that has enabled the generation of human iPSCs from adult tissue [69], coupled with recent advances in genome-editing technology [70,71,72], now allows us to investigate cellular aspects of human neurodegenerative disease in vitro. This not only potentially circumvents the dependence on scarce primary patient tissue and animal models to develop a therapy but may also facilitate a patient-tailored approach. We can now derive human iPSCs from patients and differentiate these into various tissues to model some aspects of disease in vitro [73•, 74]. Not only do these cells offer the opportunity to model cellular phenotypes but also provide a platform for high throughput drug screening and cell transplantation studies.
While it is not possible to model all aspects of a condition using iPSC-based disease modelling, it can be a very powerful tool for investigating cellular pathology. A major limitation, however, is the differentiation and phenotypic variability that is observed even in human iPSCs derived from the same donor [75, 76]. This is exacerbated further when comparing between unrelated hPSC lines. Even if the cellular phenotype of a given mutation is strong and highly penetrant, it may be lost due to genetic and epigenetic background differences [75,76,77,78,79]. A powerful approach to overcome this hurdle is to use gene editing tools that enable precise editing of endogenous hPSC genomic sequences [80]. In this scenario, gene editing can be used to correct the disease-causing mutation in a patient-derived iPSC line to generate a pair of isogenic iPSC lines that differ only in the disease-causing mutation, providing a less variable and more faithful disease modelling system. Alternatively, the same technology could be used, for example, to insert disease-causing monogenic mutations in unaffected hPSCs to generate isogenic pairs for disease modelling. The former option has the advantage that for recessive diseases only one allele needs to be repaired, whereas the latter strategy would need both alleles to be mutated. However, the latter approach would have the added advantage of reducing costs and research time, as obtaining patient samples and generating iPSCs is both costly and time consuming. These gene editing strategies will prove invaluable for studying human biology and disease.
In one of the first human iPSCs studies to model retinal disorders, Gamm and colleagues focused on Best disease, an inherited retinal dystrophy of the macula that leads to progressive and irreversible central vision loss [81]. In this disease, mutations in the RPE gene bestrophin 1 (BEST1) result in an accumulation of waste products from shed photoreceptor OS in the RPE cells, resulting in RPE dysfunction and secondary photoreceptor death. In this study, human iPSCs were derived from both patients and their unaffected siblings and differentiated into functional RPE cells. Affected iPS cell-derived RPE cells displayed delayed rhodopsin protein degradation after photoreceptor OS phagocytosis, compared to unaffected iPS cell-derived RPE. These findings indicated, for the first time in a human iPS cell-derived RPE cell, that BEST1 mutations cause defective photoreceptor OS handling [81]. In another iPS disease modelling study, Stone and colleagues modelled disease resulting from usherin (USH2A) mutations, which lead to autosomal recessive RP [82]. They derived iPSCs from a patient with a USH2A mutation and differentiated the resulting cells towards a photoreceptor fate, comparing their results to unrelated, unaffected iPSC-derived photoreceptors. The patient-derived USH2A mutant photoreceptors had upregulation of GRP78 and GRP94, suggesting that mutations in USH2A can cause endoplasmic reticulum stress, which occurs frequently in neurodegenerative diseases [82]. In a study by Cheetham and colleagues, iPSCs derived from a healthy individual and a patient bearing an LCA-causing mutation in CEP290 were differentiated into fibroblasts, RPE and photoreceptors [83]. They found that CEP290-LCA-derived fibroblasts and photoreceptors showed a reduction in the number of ciliated cells, as well as cilia length, in line with the fact that CEP290 has been shown to be important in regulating cilia assembly and development [84, 85]. The phenotype could be rescued by treating the CEP290-LCA cells with antisense morpholino oligonucleotides, which blocked the aberrant splicing of CEP290 [83].
The first reported studies demonstrate the potential of iPSC retinal disease modelling. However, there are some key issues to consider when interpreting the results and setting up future studies. Robust methods of quantification and, ideally, a comparison of several cell lines should be used; this is of even greater importance when isogenic controls are not available. The high variability in differentiation capacities between different ESCs and/or iPSCs is well documented. Even when retinal organoids are derived from the same iPSC line at the same time and are cultured under the same conditions, they can reach maturity at different times. In addition, some organoids may fail to differentiate completely, while others differentiate successfully. Such differences may arise because each organoid is likely to have different cell type content and cell-to-cell interactions. In light of such variations, comparing subtle phenotypic changes may be problematic. Repeated batch differentiation, coupled with robust quantification approaches, such as western blotting, qRT-PCR and/or RNAseq and FACS analysis will be key to obtaining meaningful and consistent results. These approaches will allow analysis of specific cell types instead of whole organoid analysis, where variation in cellular composition may create or obscure a phenotype. Variability can also be minimised by using optimal controls. For example, Gamm and colleagues used iPSCs derived from unaffected siblings as controls [81]. While the siblings may not have an identical genetic and epigenetic make-up, it is a more reliable comparison than comparing iPSC-derived retinal organoids from two unrelated individuals as used in the other studies described here [82, 83]. The optimal control, especially if the disease to be modelled is not highly penetrant, would be the same patient-derived iPSC line but gene edited to correct the disease-causing mutation (i.e. isogenic pairs). Unfortunately, the gene editing technology required to generate such isogenic controls with relative ease, CRISPR/Cas9 gene editing, has only been available for the past 4 years [70,71,72].
Stem Cells and Gene Editing
Genome editing technologies have been in the field since the 1990s (reviewed in [80]). Site-specific nucleases including zinc fingers (ZFN), transcription activator-like effectors (TALENS) and Cas9 proteins allow the introduction of precise double-strand breaks and gene modifications within the genome. Once one of these nucleases generates a DNA cut, the cell’s DNA repair mechanism is activated to avoid cell death, repairing the DNA break either by (error-prone) non-homologous end joining (NHEJ) or by (error-free) homology-directed recombination (HDR) (Fig. 1) [86,87,88,89,90,91,92]. NHEJ-mediated gene editing typically leads to the introduction of insertions and deletions (indels) in the genome, resulting in frameshift mutations that will disrupt full-length protein production through generation of a stop codon or result in a gene knockout, depending where the nucleases have created the DNA cut. On the other hand, HDR-mediated gene editing, in the presence of a homologous DNA template, can be used to modify the target DNA area with high precision. In the disease modelling context, the power of HDR-mediated gene editing can be harnessed by transfecting cells with vectors encoding one of these nucleases together with an engineered homologous DNA template that contains the desired genetic change (Fig. 1). While ZFN and TALENS are highly customizable DNA-binding proteins and can be harnessed to drive sequence-specific DNA targeting [93,94,95,96], engineering these proteins to bind to specific DNA targets, as well as achieving their robust delivery for this purpose, can be laborious and technically challenging [97]. By contrast, Cas9 nucleases are guided by two short sequences of RNA, a specificity-determining guide RNA sequence, CRISPR RNA (crRNA), and a transactivating crRNA (tracrRNA), which serves as scaffold for the Cas9 protein to form a complex (i.e. a protein:RNA complex) that forms Watson-Crick base pairs with the complementary DNA target sequence, resulting in a site-specific double-strand break [71, 72, 98] (Fig. 1). Advances in the field of CRISPR/Cas9 have rapidly led to the establishment of engineered Cas9 plasmid-based systems, where a Cas9 can be combined with a single chimeric guide RNA (gRNA), a fusion of the tracrRNA:crRNA duplex, for genome editing in eukaryotic cells at any genomic locus of interest [99,100,101]. While the CRISPR/Cas9 system is easier to use than its previous counterparts (i.e. TALENS and ZFN), some key issues still remain with regard to HDR-mediated gene editing, these include Cas9 plasmid integration, off-targeting and gene editing efficiency. For example, choosing between a plasmid-based approach or a ribonucleoprotein (RNP) CRISPR/Cas9 approach, where Cas9 protein forms a complex with synthetic gRNA [102, 103], is a key decision as each has different advantages and disadvantages. In the case of precise HDR-mediated gene editing for human iPSC disease modelling, either approach could be used. Plasmid-based gene editing allows for reporter expression which, unlike an RNP approach, will allow enrichment for the population containing the Cas9 gene editing toolkit. The use of an all-in-one plasmid approach where the reporter (e.g. puromycin or enhanced green fluorescence protein, EGFP), Cas9, gRNAs and repair template (i.e. donor template for HDR-mediated repair) are present on a single vector [100, 104] is also advantageous, as it provides obvious delivery advantages over a two or three plasmid system, where a given target cell may not receive all necessary plasmids to carry out gene editing. However, it is important to take into consideration the reporter or selection marker of choice as up to 30% plasmid background integration has been reported to occur when using a drug resistance cassette [105]. In some studies, use of fluorescent reporters such as EGFP may be preferable as FACS sorting could be used to isolate the EGFP-positive cell population (i.e. transduced cells) and, upon repeated passaging, to isolate the EGFP-negative population (i.e. transduced and free of plasmid integration). The disadvantages of using a plasmid approach, however, include that the plasmid will take 6–12 h to express the Cas9 gene editing tool kit, and it will remain in most cells for over 72 h, which means the Cas9 will remain active during this time, making off-targeting more likely [102]. If CRISPR/Cas9 gene editing is used for clinical applications, detailed evaluation of undesirable off-target modifications will be essential. Since mismatches at the 5′ end of the gRNAs are tolerated, the use of the wild-type Cas9 can lead to unintended off-target effects [106,107,108]. To address this issue, investigators have engineered modified or “evolved” Cas9 proteins [109, 110]. Among these, Cas9 nickase (Cas9n) carries a catalytic amino acid substitution (D10A) in the conserved RuvC nuclease domain, which converts this enzyme into a nickase meaning that Cas9n can only cleave one strand of DNA [110]. With this enzyme, the two gRNAs that are adjacent on opposite strands of the target site, coupled with two Cas9n molecules, would reduce off-targeting in two ways. First, since single-strand breaks are quickly repaired by the error-free base excision repair mechanism, genome integrity is maintained [111]. Second, off-targeting is greatly reduced by the two gRNAs that the Cas9n system needs to generate a double-strand break, since the likelihood of the two different gRNAs being complementary to other regions of the genome and close enough to each other to drive Cas9n-based cleavage is very low. Using the Cas9n system, Zhang and colleagues found that off-targeting was reduced 50–1500-fold. An additional approach to reduce off-targeting is to shorten the length of the gRNAs; this may reduce Cas9 activity but it greatly reduces off-targets mutations [106, 108].
The Cas9n RNP approach is an alternative way to gene edit using the CRISPR/Cas9 system for in vitro disease modelling. This method circumvents the problem of integration in plasmid-based systems and significantly reduces off-targeting, since the Cas9 RNP complex is rapidly degraded in cells within 24 h when delivered directly [102, 103]. This means that DNA breaks would occur for a shorter period of time and since there is no plasmid in this approach, there is no risk of integration. Furthermore, unlike the plasmid approach, since the Cas9 RNP is an active nuclease complex, it starts to gene edit as soon as it is transfected into the cells. Cas9 RNP approaches have been shown to be highly efficient in introducing indels and HDR-mediated precise gene edits [102, 103], although to our knowledge an all-in-one plasmid vs RNP approach has not been directly compared for the same target gene using the same gRNAs.
The importance of generating isogenic iPSC line pairs has been discussed earlier. Jaenisch and colleagues showed that gene editing can be used to generate isogenic pairs (e.g. affected and repaired) from human iPSCs or ESCs, leading to two cell lines that have the same genetic and epigenetic background and differ only in the disease-causing mutation [112]. In this study, ZFNs were engineered to target the α-synuclein (SNCA) locus, a gene commonly mutated in Parkinson’s disease. Using different gene editing strategies, they introduced two common mutations of SNCA in unaffected ESCs or corrected the mutation in a patient-derived iPSC line [112]. Co-electroporation of the ZFN expression plasmids and donor constructs, followed by selection of the transfected cells, led to a genome editing efficiency of ∼0.9% (3 out of 336 screened clones where targeted at the correct locus). Furthermore, using a positive and negative selection approach, they obtained a maximum efficiency of ∼22% (9 out of 41 screened clones correct for the desired mutation). The resulting cells were karyotypically normal, maintained pluripotency and were able to differentiate into dopaminergic neurons [112]. This study represents one of the first reports of patient-derived iPSCs and wild-type human ESCs being gene edited to create isogenic pairs for disease modelling. This not only represents a significant progress for in vitro disease modelling but also a major advancement towards human iPSC-based cell replacement therapies.
In the context of in vivo applications in the retina, an RNP approach may not be the most suitable, as achieving efficient delivery of the RNP complex into retinal cells may prove difficult. Given the proven efficacy of viral vector-mediated gene transfer to the retina, a gene-based approach, where the Cas9 tool kit is packaged in an AAV vector, may be easier. However, AAV vectors can only package approximately ∼5 Kb of insert and the Streptococcus pyogenes Cas9 (SpCas9) DNA is about 4.2 Kb, leaving little or no room for a reporter or gRNA and repair template. Different approaches have been taken to overcome this problem in other cell types. Zhang and colleagues used a dual AAV system approach to deliver SpCas9 and gRNAs to disrupt gene function in dividing mouse brain cells in vivo [113]. The same group later demonstrated that using the SpCas9 orthologue, Staphylococcus aureus Cas9 (SaCas9), which is 3.2 Kb and therefore approximately 1 Kb smaller than SpCas9 can be packaged into a single AAV system along with gRNAs and can introduce high levels of gene disruption in mouse liver cells in vivo [114]. Similarly, SaCas9 has been used in both a dual and a single AAV system, with various gRNAs, to target mouse muscle cells in vivo [115]. Recently, Hewitt and colleagues attempted a dual AAV2 CRISPR/Cas9 system approach for disrupting the expression of yellow fluorescent protein (YFP) in retinal ganglion cells of a Thy1-YFP transgenic mouse model [116]. They managed to reduce the number of YFP-positive cells by 84%, apparently without affecting normal retinal function. This proof-of-concept study for NHEJ-based gene editing in the retina, together with the in vivo AAV-based gene editing studies in other tissues, suggests that in vivo retinal gene editing may be achievable at efficiencies high enough for in vivo retinal therapy in the future (Fig. 1). While using AAV to deliver the necessary CRISPR components for efficient gene editing is challenging due to size constraints, other viral vectors are able to accommodate larger DNA inserts. Lentiviral vectors, for example, can package approximately 9 kb of insert DNA that would be large enough to accommodate both a large Cas9 nuclease (e.g. SpCas9) and the gRNA cassettes. One such approach has been used to carry out gene editing in RPE cells where VEGF-A was disrupted in vitro in human cells [117]. A similar approach could be used in vivo for targeted gene disruption in the RPE but not for targeting photoreceptor as lentiviral vectors do not transduce these cells efficiently. However, a major problem that still remains to be addressed is how to achieve efficient and accurate HDR-based gene editing in the retina if most of its cells are post-mitotic and largely lack HDR repair.
Together, these studies suggest that disease modelling of a variety of inherited retinal diseases using iPSCs is a realistic prospect. However, the appropriate choice of control cell line and gene editing tool kits to detect subtle phenotypic changes in affected cell types will be critical for successful disease modelling. Regarding in vivo gene editing in the retina, packaging the whole CRISPR/Cas9 gene editing tool kit in a single AAV vector for delivery will likely lead to the highest levels of transduction efficiency and gene disruption in the retina. However, an efficient method to carry out precise DNA substitutions in post-mitotic retinal cells remains to be elucidated.
Summary and Future Challenges
hPSCs represent a powerful tool for understanding human retinal development, retinal disease modelling, drug discovery and for developing cell transplantation therapies. However, to achieve these successfully, it is necessary to consider a number of requirements. A significant amount of further work is required to establish robust and reproducible protocols for the efficient generation of retinal organoids from hPSC sources. Of particular importance is the ability to generate fully mature hPSC-derived photoreceptors including OS in vitro. This may necessitate the use of bioreactors, co-cultures of RPE cells and/or the use of bioscaffolds. Faithful disease modelling will benefit from the use of isogenic pairs of iPSC lines, as well as robust photoreceptor quantification methods and improved retinal organoid differentiation. Reducing the variability between differentiations remains a major challenge. Generating isogenic controls for the affected cell type should significantly reduce the inherent variability that is introduced by differences in epigenetic and genetic background when using iPSCs from different sources. Methods to assess the phenotype should be quantitative and robust. Patient-specific iPSCs are not only a powerful tool for disease modelling for inherited retinal degeneration but may in the future also become a source of cells for photoreceptor transplantation. Two key aspects we need to consider for cell transplantation studies are the optimal developmental stage of the iPSC-derived retinal tissue to transplant [35, 41, 118], and the genetic correction of the patient’s affected cell type before delivery. Recently, a CD surface marker panel was described for murine ESC-derived photoreceptors. This panel allowed for the isolation and purification of rod photoreceptor cells at the optimal stage of development for transplantation [40, 61]. Promising preliminary studies in human retinal tissue further indicated that a similar panel should be identifiable for hPSC-derived retinal tissue [61], opening the way to isolating photoreceptors for cell transplantation therapies in humans. If iPSCs from patients are to be used as the donor source, it will also be necessary to repair the inherited defect prior to transplantation. CRISPR/Cas9 gene editing is likely to play a key role here. For clinical use, a RNP CRISPR Cas9n gene editing approach is likely to be most advantageous, as it offers high gene editing efficiency and minimum off-targeting effects. A stringent screening of the resulting iPS-derived photoreceptors will be required prior to transplantation; karyotyping, tumorigenicity and whole genome sequencing screens will be necessary to ensure safety in phase I clinical trials. Takahashi and colleagues initiated the first human RPE iPSC-derived phase I clinical trial for neovascular AMD in 2014. The first subject in the trial received autologous iPSC-derived RPE, while subsequent subjects received allogenic iPSC-derived RPE. While several studies have recently shown that hESC-derived RPE cells are safe following transplantation into patients in phase I clinical trials [119,120,121], some concerns were raised in the study by Takahashi and colleagues about the stability of the genome of the iPSC-derived RPE cells used for sheet transplantation, causing the trial to be temporarily halted. Regardless, this landmark study served as proof-of-principle to show that iPSC-derived cell therapy in humans is possible.
The major advantage of patient-specific, gene-corrected, autologous iPSC-derived cells over hESC-derived cells is reduced immunogenicity following transplantation. However, developing clinical-grade autologous iPSC-derived cells for each individual is likely to be prohibitively expensive for the foreseeable future and alternative strategies are therefore required. In an effort to address this issue, Takahashi and colleagues have recently shown that non-human primate iPSC-derived RPE, transplanted into allogenic major histocompatibility complex (MHC)-matched animals, survived without evidence of rejection while iPSC-derived RPE transplanted into MHC-mismatched animals resulted in immune rejection [122••]. Furthermore, Shinja Yamanaka, Nobel Prize winner for his discovery on iPSCs, is developing a bank of clinical grade iPSC lines that are homozygous for the common Japanese MHC types. This should allow MHC-matched patients to be treated with the same iPSC source, greatly reducing costs and variability of differentiation.
HDR-mediated gene editing in vivo may have potential in the future to treat inherited retinal degenerations, particularly those caused by dominant mutations. Successful in vivo gene disruption via NHEJ using CRISPR/Cas9 in an AAV system has been shown in several tissues. However, HDR-mediated gene editing for precise DNA modifications in post-mitotic neurons remains to be achieved. If HDR-mediated gene editing in post-mitotic retinal cells is achieved using a single AAV system, another aspect to consider will be the requirement to use an inducible CRISPR/Cas9 system where induction of gene editing can be stimulated with a drug and consequently switched off after correction, since long-term expression of Cas9 system could lead to an increase off-targeting of the genome.
Regardless of the undoubted ahead challenges, these are exciting times and the landmark studies discussed in this review open the door to widespread application of hPSC-based technology and gene editing to advance our understanding and treatment of retinal disorders.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Visual impairment and blindness Fact Sheet N°282. Retrieved 24 Oct 2016. World Health Organization. 2014.
Quartilho A, Simkiss P, Zekite A, Xing W, Wormald R, Bunce C. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye (Lond). 2016;30(4):602–7. doi:10.1038/eye.2015.288.
Huang Y, Enzmann V, Ildstad ST. Stem cell-based therapeutic applications in retinal degenerative diseases. Stem Cell Rev. 2011;7(2):434–45. doi:10.1007/s12015-010-9192-8.
Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29(5):335–75. doi:10.1016/j.preteyeres.2010.03.004.
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–809. doi:10.1016/S0140-6736(06)69740-7.
Minassian DC, Reidy A, Lightstone A, Desai P. Modelling the prevalence of age-related macular degeneration (2010-2020) in the UK: expected impact of anti-vascular endothelial growth factor (VEGF) therapy. Br J Ophthalmol. 2011;95(10):1433–6. doi:10.1136/bjo.2010.195370.
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012;96(5):752–6. doi:10.1136/bjophthalmol-2011-301109.
Taylor HR. Diabetic retinopathy. Clin Exp Ophthalmol. 2005;33(1):3–4. doi:10.1111/j.1442-9071.2004.00967.x.
Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol. 2012;3:296. doi:10.3389/fimmu.2012.00296.
Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69.
Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74(2):179–85.
Mullins RF, Faidley EA, Daggett HT, Jomary C, Lotery AJ, Stone EM. Localization of complement 1 inhibitor (C1INH/SERPING1) in human eyes with age-related macular degeneration. Exp Eye Res. 2009;89(5):767–73. doi:10.1016/j.exer.2009.07.001.
Mullins RF, Kuehn MH, Radu RA, Enriquez GS, East JS, Schindler EI, et al. Autosomal recessive retinitis pigmentosa due to ABCA4 mutations: clinical, pathologic, and molecular characterization. Invest Ophthalmol Vis Sci. 2012;53(4):1883–94. doi:10.1167/iovs.12-9477.
Warrington Jr KH, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119(6):571–603. doi:10.1007/s00439-006-0165-6.
Smith AJ, Bainbridge JW, Ali RR. Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther. 2012;19(2):154–61. doi:10.1038/gt.2011.161.
Trapani I, Banfi S, Simonelli F, Surace EM, Auricchio A. Gene therapy of inherited retinal degenerations: prospects and challenges. Hum Gene Ther. 2015;26(4):193–200. doi:10.1089/hum.2015.030.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–9. doi:10.1056/NEJMoa0802268.
Edwards TL, Jolly JK, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374(20):1996–8. doi:10.1056/NEJMc1509501.
Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135(3):327–43. doi:10.1007/s00439-016-1637-y.
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–90. doi:10.1089/hum.2008.107.
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi:10.1001/archophthalmol.2011.298.
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi:10.1016/S0140-6736(13)62117-0.
Maguire AM, Simonelli F, Pierce EA, Pugh Jr EN, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi:10.1056/NEJMoa0802315.
Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372(20):1887–97. doi:10.1056/NEJMoa1414221.
Jacobson SG, Cideciyan AV, Roman AJ, Sumaroka A, Schwartz SB, Heon E, et al. Improvement and decline in vision with gene therapy in childhood blindness. N Engl J Med. 2015;372(20):1920–6. doi:10.1056/NEJMoa1412965.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–605. doi:10.1016/S0140-6736(09)61836-5.
Hermonat PL, Quirk JG, Bishop BM, Han L. The packaging capacity of adeno-associated virus (AAV) and the potential for wild-type-plus AAV gene therapy vectors. FEBS Lett. 1997;407(1):78–84.
Hirsch ML, Green L, Porteus MH, Samulski RJ. Self-complementary AAV mediates gene targeting and enhances endonuclease delivery for double-strand break repair. Gene Ther. 2010;17(9):1175–80. doi:10.1038/gt.2010.65.
Wang Y, Ling C, Song L, Wang L, Aslanidi GV, Tan M, et al. Limitations of encapsidation of recombinant self-complementary adeno-associated viral genomes in different serotype capsids and their quantitation. Hum Gene Ther Methods. 2012;23(4):225–33. doi:10.1089/hgtb.2012.090.
Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18(1):80–6. doi:10.1038/mt.2009.255.
Han Z, Conley SM, Naash MI. Gene therapy for Stargardt disease associated with ABCA4 gene. Adv Exp Med Biol. 2014;801:719–24. doi:10.1007/978-1-4614-3209-8_90.
Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2016; doi:10.1136/bjophthalmol-2016-308823.
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426(6966):570–4. doi:10.1038/nature02166.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. doi:10.1038/nature09941. A groundbreaking pioneering study describing 3D retinal organoid cell culture differentiation.
Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31(8):741–7. doi:10.1038/nbt.2643.
Barber AC, Hippert C, Duran Y, West EL, Bainbridge JW, Warre-Cornish K, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110(1):354–9. doi:10.1073/pnas.1212677110.
Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86(4):691–700. doi:10.1016/j.exer.2008.01.018.
Eberle D, Schubert S, Postel K, Corbeil D, Ader M. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci. 2011;52(9):6462–71. doi:10.1167/iovs.11-7399.
Lakowski J, Baron M, Bainbridge J, Barber AC, Pearson RA, Ali RR, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19(23):4545–59. doi:10.1093/hmg/ddq378.
Lakowski J, Han YT, Pearson RA, Gonzalez-Cordero A, West EL, Gualdoni S, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29(9):1391–404. doi:10.1002/stem.694.
MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–7. doi:10.1038/nature05161.
Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi:10.1038/nature10997.
West EL, Gonzalez-Cordero A, Hippert C, Osakada F, Martinez-Barbera JP, Pearson RA, et al. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30(7):1424–35. doi:10.1002/stem.1123.
Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, et al. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016;6:29784. doi:10.1038/srep29784.
Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–9. doi:10.1016/j.stem.2008.10.015.
Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, et al. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science. 2016;353(6294):aaf3646. doi:10.1126/science.aaf3646.
Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6(4):e18992. doi:10.1371/journal.pone.0018992.
Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(34):12769–74. doi:10.1073/pnas.0601990103.
Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5(1):e8763. doi:10.1371/journal.pone.0008763.
Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30(4):673–86. doi:10.1002/stem.1037.
Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29(8):1206–18. doi:10.1002/stem.674.
Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106(39):16698–703. doi:10.1073/pnas.0905245106.
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85. doi:10.1016/j.stem.2012.05.009.
Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215–24. doi:10.1038/nbt1384.
• Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A. 2014;111(23):8518–23. doi:10.1073/pnas.1324212111. This study shows how retinal organoid differentiation can be achieved taking advantage of the ability of human pluripotent stem cells to spontaneously differentiate towards the neural lineage without the use of expensive SMAD inhibitors and using a 2D to 3D cell differentiation approach.
•• Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi:10.1038/ncomms5047. This study demonstrates how human pluripotent stem cells can be differentiated towards mature photoreceptors that are responsive to light using a 3D to 2D to 3D differentiation approach that uses no SMAD inhibitors.
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. doi:10.1038/nbt.1529.
Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23(9):1234–41. doi:10.1634/stemcells.2005-0110.
Ovando-Roche P, Yu JS, Testori S, Ho C, Cui W. TRF2-mediated stabilization of hREST4 is critical for the differentiation and maintenance of neural progenitors. Stem Cells. 2014;32(8):2111–22. doi:10.1002/stem.1725.
•• Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113(1):E81-90. doi:10.1073/pnas.1512590113. This report shows how human embryonic stem cell-derived retinal organoid tissue transplanted into nude rats recipient retina induces the maturation of photoreceptor outer segments. precursor photoreceptors into nude rats allows the mature photoreceptor outer segments previously not possible in vitro.
Lakowski J, Gonzalez-Cordero A, West EL, Han YT, Welby E, Naeem A, et al. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells. 2015;33(8):2469–82. doi:10.1002/stem.2051.
Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24(2):246–57. doi:10.1634/stemcells.2005-0009.
Lamba DA, Reh TA. Microarray characterization of human embryonic stem cell—derived retinal cultures. Invest Ophthalmol Vis Sci. 2011;52(7):4897–906. doi:10.1167/iovs.10-6504.
Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24(2):274–83. doi:10.1634/stemcells.2005-0059.
Kelava I, Lancaster MA. Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev Biol. 2016; doi:10.1016/j.ydbio.2016.06.037.
Kempf H, Olmer R, Kropp C, Ruckert M, Jara-Avaca M, Robles-Diaz D, et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports. 2014;3(6):1132–46. doi:10.1016/j.stemcr.2014.09.017.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi:10.1126/science.1247125.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9. doi:10.1038/nature12517.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi:10.1016/j.cell.2007.11.019.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. doi:10.1126/science.1231143.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. doi:10.1126/science.1225829.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. doi:10.1126/science.1232033.
• Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–97. doi:10.1016/j.cell.2016.05.082. An excellent review describing how the power of human pluripotent stem cells and gene editing has been harnessed to generate different tissues of the human body and model disease in vitro.
Duong TT, Vasireddy V, Mills JA, Bennett J. Retinas in a dish peek into inherited retinal degeneration. Cell Stem Cell. 2016;18(6):688–9. doi:10.1016/j.stem.2016.05.021.
Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144(3):439–52. doi:10.1016/j.cell.2010.12.032.
Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):279–86. doi:10.1038/nbt.1783.
Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12(6):656–68. doi:10.1016/j.stem.2013.05.016.
Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat Neurosci. 2013;16(7):780–9. doi:10.1038/nn.3425.
Sterneckert JL, Reinhardt P, Scholer HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15(9):625–39. doi:10.1038/nrg3764.
Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–34. doi:10.1038/nrg3686.
Singh R, Shen W, Kuai D, Martin JM, Guo X, Smith MA, et al. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22(3):593–607. doi:10.1093/hmg/dds469.
Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. elife. 2013;2:e00824. doi:10.7554/eLife.00824.
Parfitt DA, Lane A, Ramsden CM, Carr AJ, Munro PM, Jovanovic K, et al. Identification and correction of mechanisms underlying inherited blindness in human iPSC-derived optic cups. Cell Stem Cell. 2016;18(6):769–81. doi:10.1016/j.stem.2016.03.021.
Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17(23):3796–805. doi:10.1093/hmg/ddn277.
Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15(2):187–97. doi:10.1016/j.devcel.2008.07.004.
Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300(5620):764. doi:10.1126/science.1079512.
Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, et al. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 2001;21(1):289–97. doi:10.1128/MCB.21.1.289-297.2001.
Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12(3):1292–303.
Liang F, Han M, Romanienko PJ, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci U S A. 1998;95(9):5172–7.
Plessis A, Perrin A, Haber JE, Dujon B. Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics. 1992;130(3):451–60.
Rouet P, Smih F, Jasin M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1994;91(13):6064–8.
Rudin N, Sugarman E, Haber JE. Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics. 1989;122(3):519–34.
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186(2):757–61. doi:10.1534/genetics.110.120717.
Gaj T, Gersbach CA, Barbas III CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi:10.1016/j.tibtech.2013.04.004.
Miller JC, Holmes MC, Wang J, Guschin DY, Lee YL, Rupniewski I, et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol. 2007;25(7):778–85. doi:10.1038/nbt1319.
Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29(2):149–53. doi:10.1038/nbt.1775.
Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi:10.1146/annurev.biophys.29.1.183.
Briner AE, Donohoue PD, Gomaa AA, Selle K, Slorach EM, Nye CH, et al. Guide RNA functional modules direct Cas9 activity and orthogonality. Mol Cell. 2014;56(2):333–9. doi:10.1016/j.molcel.2014.09.019.
Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(3):230–2. doi:10.1038/nbt.2507.
Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, Sontheimer EJ, et al. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc Natl Acad Sci U S A. 2013;110(39):15644–9. doi:10.1073/pnas.1313587110.
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–9. doi:10.1038/nbt.2501.
Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24(6):1012–9. doi:10.1101/gr.171322.113.
Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. elife. 2014;3:e04766. doi:10.7554/eLife.04766.
Sakuma T, Nishikawa A, Kume S, Chayama K, Yamamoto T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci Rep. 2014;4:5400. doi:10.1038/srep05400.
Merkle FT, Neuhausser WM, Santos D, Valen E, Gagnon JA, Maas K, et al. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell Rep. 2015;11(6):875–83. doi:10.1016/j.celrep.2015.04.007.
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–6. doi:10.1038/nbt.2623.
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–32. doi:10.1038/nbt.2647.
Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31(9):839–43. doi:10.1038/nbt.2673.
Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523(7561):481–5. doi:10.1038/nature14592.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. doi:10.1038/nprot.2013.143.
Dianov GL, Hubscher U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 2013;41(6):3483–90. doi:10.1093/nar/gkt076.
Soldner F, Laganiere J, Cheng AW, Hockemeyer D, Gao Q, Alagappan R, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146(2):318–31. doi:10.1016/j.cell.2011.06.019.
Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33(1):102–6. doi:10.1038/nbt.3055.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–91. doi:10.1038/nature14299. http://www.nature.com/nature/journal/v520/n7546/abs/nature14299.html#supplementary-information
Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407–11. doi:10.1126/science.aad5177.
Hung SS, Chrysostomou V, Li F, Lim JK, Wang JH, Powell JE, et al. AAV-mediated CRISPR/Cas Gene editing of retinal cells in vivo. Invest Ophthalmol Vis Sci. 2016;57(7):3470–6. doi:10.1167/iovs.16-19316.
Yiu G, Tieu E, Nguyen AT, Wong B, Smit-McBride Z. Genomic disruption of VEGF-A expression in human retinal pigment epithelial cells using CRISPR-Cas9 endonuclease. Invest Ophthalmol Vis Sci. 2016;57(13):5490–7. doi:10.1167/iovs.16-20296.
Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Rep. 2014;2(6):853–65. doi:10.1016/j.stemcr.2014.04.010.
Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–20. doi:10.1016/S0140-6736(12)60028-2.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–16. doi:10.1016/S0140-6736(14)61376-3.
Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep. 2015;4(5):860–72. doi:10.1016/j.stemcr.2015.04.005.
•• Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, et al. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. 2016; doi:10.1016/j.stemcr.2016.08.010. This study shows that transplanting iPSC-derived RPE allografts into MHC-matched animal models does not result in rejection suggesting that a bank of clinical grade iPSCs that are homozygous for the common Japanese MHC type may be a less costly alternative to autologous iPSC-derived tissue tranplantation. the way forward to treatMHC-matched clinical grade iPSCs may be a solution of allogenic stem cell.
Acknowledgments
This review was supported by grants from European Research Council (ERC-2012-ADG_20120314), the Medical Research Council UK (MR/J004553/1 and MR/M015815), Fight For Sight (1448/1449), the Macular Vision Research Foundation, the Special Trustees of Moorfields Eye Charity. R.A.P. is a Royal Society University Research Fellow (UF120046). R.R.A is partially funded by the Department of Health’s National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital and Institute of Ophthalmology. R.A.P is part funded by a grant from the Alcon Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Patrick Ovando-Roche declares that he has no conflict of interest.
Anastasios Georgiadis reports personal fees from MeiraGTx UK II Ltd.
Alexander J. Smith reports personal fees from MeiraGTx UK II Ltd.
Rachael A. Pearson reports grants from Alcon Research Institute.
Robin R. Ali reports personal fees from MeiraGTx UK Ltd.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Rachael A. Pearson and Robin R. Ali are joint senior authors.
This article is part of the Topical Collection on Genome Editing
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ovando-Roche, P., Georgiadis, A., Smith, A.J. et al. Harnessing the Potential of Human Pluripotent Stem Cells and Gene Editing for the Treatment of Retinal Degeneration. Curr Stem Cell Rep 3, 112–123 (2017). https://doi.org/10.1007/s40778-017-0078-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-017-0078-4