Abstract
Introduction
RA-BE-REAL has the overall aim of defining a profile of patients with rheumatoid arthritis (RA) starting baricitinib or any other targeted synthetic (ts) or any biologic (b) disease-modifying antirheumatic drug (DMARD) for the first time, and the primary objective of estimating time until discontinuation from any cause (excluding sustained response) of the initial treatment.
Methods
RA-BE-REAL is an ongoing, prospective, observational, 36-month study in patients with RA initiating treatment with baricitinib (cohort A) or any other tsDMARD or any bDMARD (cohort B) for the first time. The primary objective is to assess the time until treatment discontinuation from any cause (excluding sustained response) at 24 months, (i.e., the rate of discontinuation of initial baricitinib or ts/bDMARD). Patient profiles of each cohort are described and compared. Post-baseline data are descriptively analyzed. This manuscript presents baseline and interim (6-month) outcomes for European patients with RA participating in the global RA-BE-REAL study.
Results
Data from 1074 patients (cohort A: 509; cohort B: 565) were analyzed. For cohorts A and B, respectively, the 6-month cumulative incidence (95% confidence interval) of treatment discontinuation was 16.5 (12.9–21.1) and 23.3 (19.1–28.2), and the proportions of patients achieving remission were 25.6% and 18.5%. At baseline, mean patient age was 59.1 and 57.0 years (p = 0.010) and mean disease duration was 10.0 and 8.9 years (p = 0.047), respectively. The proportions of patients exposed to ts/bDMARDs at any time before study entry were 51.9% and 39.1%, and the proportions of patients initiated on monotherapy were 50.9% and 31.2%, respectively.
Conclusion
In real-world settings, patients with RA initiating treatment with baricitinib were older and had longer disease duration than those initiating treatment with any other tsDMARD or any bDMARD. Initial descriptive data regarding treatment discontinuation (including reasons for discontinuation), effectiveness, and treatment patterns will be enriched as the study progresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Treatment discontinuation is common among patients with rheumatoid arthritis (RA). |
The analysis characterized patients with RA receiving baricitinib (cohort A) versus another targeted synthetic (ts) or any biological (b) disease-modifying antirheumatic drug (DMARD; cohort B) for the first time, and determined time until treatment discontinuation and the effectiveness of baricitinib and any other tsDMARD or any bDMARD in real-world settings in Europe. |
What was learned from this study? |
Patients with RA initiating treatment with baricitinib were older and had a longer disease duration than those initiating treatment with any other tsDMARD or any bDMARD. |
Initial (6-month) descriptive data on treatment discontinuation revealed cumulative incidences (95% CI) of discontinuation of 16.5% (12.9–21.1) for cohort A and 23.3% (19.1–28.2) for cohort B; the main reason for discontinuation was primary nonresponse (3.3% of cohort A and 5.8% of cohort B). |
Clinical Disease Activity Index showed a mean (standard deviation) change from baseline to 6 months of −13.9 (12.5) for cohort A and −11.8 (13.2) for cohort B, with 25.6% and 18.5% of each cohort, respectively, achieving remission at 6 months. |
Over 6 months of observation, baricitinib proved to be an effective and long-lasting treatment choice for patients with RA initiating a ts/bDMARD for the first time in their treatment algorithm in real-world settings; more data will be generated with the observation of these patients up to 3 years, as per the study design. |
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease associated with pain and swelling of the joints [1]. Further, if insufficiently treated, RA can cause extra-articular manifestations and comorbidities. As a result, RA adversely affects patients’ physical functioning, work productivity, and health-related quality of life (HRQoL) [1] .
The treat-to-target concept, aiming at disease remission or low disease activity (LDA) [2, 3], is the current paradigm in the treatment of RA [4]. The timely and appropriate use of disease-modifying antirheumatic drugs (DMARDs), including the conventional synthetic (cs), biological (b), and targeted synthetic (ts) DMARDs, has contributed significantly to the achievement of these targets.
Despite these advances, treatment discontinuation is common for many patients with RA. Decreased drug maintenance rates over time have been observed for the tumor necrosis factor inhibitors (TNFis), a subset of bDMARDs, in drug registries for rheumatic diseases such as the BIOBADASER from Spain[5] or the ARTIS from Sweden[6]; similarly, declining drug maintenance rates for non-TNFis (specifically rituximab, abatacept, and tocilizumab) have been observed in registry studies in France [7]. Moreover, a recent systematic review and meta-analysis of world drug registries and health care databases reported that the TNFi discontinuation rates increased from 21% at 6 months to 52% at 48 months [8]. Loss of efficacy appears to be the main reason for discontinuing bDMARDs, followed by physician preference, safety, patient preference, and no access to treatment [9]. By contrast, the main reason for discontinuation of methotrexate, the most commonly used csDMARD [10], appears to be poor tolerability [11]. These findings pose a challenge for the management of RA, although the route of drug administration does not appear to be a dominant factor for drug adherence or persistence in RA [12].
Baricitinib is an oral, reversible, and selective Janus kinase (JAK)1/JAK2 inhibitor[13, 14] approved in more than 70 countries for the treatment of adults with moderately to severely active RA [15]. Baricitinib has shown efficacy and safety in treating patients with moderate-to-severe RA in an extensive program of phase II and III clinical studies [16]. In one of these studies, which included patients with active RA and inadequate response to methotrexate, treatment with oral baricitinib 4 mg showed superiority versus adalimumab (both given in addition to methotrexate), as evidenced by the American College of Rheumatology 20 response rates and the Disease Activity Scores for 28 joints with the use of high-sensitivity C-reactive protein at 12 weeks [17]. Baricitinib was granted marketing authorization in Europe in 2017. However, data on its effectiveness and discontinuation rate versus other DMARDs are sparse, comprising only a single study from Japan[18] and analyses of the Swiss registry for rheumatic diseases [19, 20].
The ongoing, real-world RA-BE-REAL study is a 3-year study primarily assessing the time until discontinuation (for any reason except sustained clinical response) in patients with RA initiating treatment with baricitinib or any other tsDMARD or any bDMARD for the first time in their treatment algorithm. This interim analysis presents the study outcomes for European patients enrolled in RA-BE-REAL at 6 months, and is, as per the statistical analysis plan, mainly descriptive.
Methods
Study Design and Patient Population
RA-BE-REAL is an ongoing, prospective, observational study conducted in five European countries (Germany, France, UK, Spain, and Italy) and Australia, Canada, and Saudi Arabia. The study commenced in October 2018, and completion is expected in October 2024. Patient enrollment for the European countries was completed in March 2020. This interim analysis includes patient data from the European countries over the first 6 months of follow-up.
The design of the RA-BE-REAL study has been previously published (Supplementary Fig. 1 [21]). Briefly, patients with RA initiating treatment with baricitinib 2 or 4 mg once daily (cohort A) or any other tsDMARD or any bDMARD (cohort B) for the first time at any point in the treatment algorithm in routine clinical care are followed-up for approximately 36 months (observation period). During this period, study visits (data collection points) are scheduled at enrollment (baseline) and post-baseline at approximately 3, 6, 12, 18, 24, and 36 months, as per routine clinical care; the defined window period for each visit is ± 4 weeks. The participating investigators are physicians involved in the routine clinical care of patients with RA; treatment initiation with baricitinib or another tsDMARD or any bDMARD, and any treatment changes during the observation period, are solely at the discretion of the participating physicians in line with locally applicable guidelines and clinical routine.
Patients were eligible for inclusion if they were aged 18 years or older, met the criteria for RA according to their treating physician, and initiated treatment with baricitinib or another tsDMARD or any bDMARD for the first time at any point in their treatment algorithm. Although eligible patients could have previously received other tsDMARDs or bDMARDs at any point in their treatment algorithm, the specific tsDMARD or bDMARD initiated at study entry was not previously prescribed. Patients who simultaneously participate in any other study that includes an investigational drug or procedure, either at entry or during the observation period of the current study were excluded.
The study is being conducted in accordance with the ethical principles of the Declaration of Helsinki [22] and Good Clinical Practice guidelines and the applicable laws and regulations of the five European countries. All included patients provided written informed consent before study entry.
Study Objectives and Endpoints
The overall aim of RA-BE-REAL is to define a patient profile of patients with RA starting baricitinib, or any other tsDMARD or any bDMARD for the first time, describing patient characteristics, clinical and patient-reported outcomes, health care resource utilization and costs, as well as describing how these treatments are used in a real-world setting by assessing treatment patterns. As such, the primary objective is to observe time until discontinuation of their initial baricitinib, tsDMARD, or bDMARD treatment for any causes (excluding sustained clinical response) within each cohort over 24 months of follow-up. The cumulative incidence of patients who have discontinued treatment at 24 months per cohort will be estimated.
A secondary objective and important aim of the study is to describe the patients’ baseline demographic and clinical characteristics (e.g., duration of initial RA diagnosis, previous RA treatment and reasons for change, concomitant diseases). Other secondary objectives are to describe the RA treatment patterns with baricitinib or any other tsDMARD or any bDMARD, including the use of concomitant background RA medication, and the reasons for treatment discontinuation.
Secondary endpoints, assessed at 6 months, are the mean change from baseline in the Clinical Disease Activity Index (CDAI) and the proportions of patients achieving remission and LDA by CDAI and patient-reported outcomes (PROs). Remission, LDA, moderate, and severe disease activity are defined as CDAI score ranges of 0.0–2.8, 2.9–10.0, 10.1–22.0, and 22.1–76.0, respectively [23]. The PROs assessed include (i) RA-related pain, using a 0–100 mm visual analog scale (VAS); (ii) physical function, using the Health Assessment Questionnaire-Disability Index (HAQ-DI) [24, 25]; and (iii) quality of life, using the EQ-5D-5L [26, 27]. Other study variables assessed are the swollen and tender joint counts and the physician’s and patient’s global assessments.
Lastly, health care resource utilization is being assessed by recording physician visits, hospitalizations, and diagnostic and imaging tests for each country.
The intention of this manuscript is to present the results of the interim analyses descriptively, owing to the immaturity of the data collected during the study follow-up of up to 6 months.
Statistical Analysis
All patients from the participating European countries who fulfilled the inclusion and did not meet any of the exclusion criteria, as defined in the statistical analysis plan of the study, were included in the current analyses. Post-baseline analyses were descriptive and were performed by treatment cohort and overall; for RA treatment discontinuations and clinical outcomes at 6 months, cohort B analyses were also performed by DMARD mode of action (i.e., TNFi and non-TNFi bDMARDs and any other tsDMARDs).
In RA-BE-REAL, the reasons for discontinuation are categorized as primary nonresponse, secondary loss of response, adverse event, sustained clinical response, changes to reimbursement criteria or health insurance, noncompliance, patient or physician decision, cannot afford medication, or “other.” For the primary endpoint, the time to treatment discontinuation is calculated from the date of initiation of baricitinib or another tsDMARD or any bDMARD until the date of treatment discontinuation (for any reason excluding sustained response), as reported in the patient’s medical record. If the discontinuation date is not available for patients who switched treatment to a new ts/bDMARD, the start date of the new ts/bDMARD is considered the discontinuation date of the initial ts/bDMARD. Also, if the initial ts/bDMARD is not used for two or more consecutive 6-month periods, or two or more consecutive observations are missing, discontinuation is assumed at the end of the 6-month period when the initial ts/bDMARD is last reported. An additional 6-month gap is allowed for rituximab as its dosing schedule is every 6–12 months. Of note, the short (6 months) observation period of this interim RA-BE-REAL report precluded analysis of discontinuations as described above; therefore, all data were analyzed “as observed.”
As defined in the statistical analysis plan, descriptive statistics were used to analyze post-baseline patient data, including RA treatment discontinuation, CDAI values, and proportions of patients achieving LDA or remission, and the HAQ-DI, EQ-5D-5L, and RA-related pain VAS scores at each visit. Continuous variables are presented with mean values and standard deviations (SDs). Categorical variables are presented with absolute numbers and relative frequencies. The t-test and the chi-squared test were used for the comparison of continuous and categorical variables, respectively, only for the baseline data, to evaluate whether the two cohorts differed in terms of those characteristics and to help determine whether any patterns of physician choice of treatment can be detected based on individual patient and disease characteristics. Statistical significance was set at the level of p < 0.05. The time to discontinuation was estimated using Kaplan–Meier analysis for the overall cohorts, and subgroups of the baricitinib cohort. For this analysis, the cumulative incidence of discontinuation was calculated at 6 months and presented with 95% confidence intervals (CIs). Patients for whom the treatment discontinuation date was missing were censored at the last observation available. Lastly, health care resource utilization (HCRU) analyses were performed at the country level only, using descriptive statistics. For each cohort, the RA-related HCRU was assessed by combining physician- and patient- (or caregiver-) reported HCRU data.
The statistical analyses used SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Patient Disposition and Baseline Characteristics
Overall, 1074 patients initiated treatment with baricitinib 2 or 4 mg (cohort A, 509 patients) or with any other tsDMARD or any bDMARD (cohort B, 565 patients) and were included in this 6-month interim analysis. The enrollment period lasted from October 2018 to March 2020. At the country level, 423 of the included patients were from Germany (cohort A: 240; cohort B: 183), 221 from France (cohort A: 103; cohort B: 118), 191 from Italy (cohort A: 80; cohort B: 111), 159 from the UK (cohort A: 55; cohort B: 104), and 80 from Spain (cohort A: 31; cohort B: 49).
The patients’ baseline demographic characteristics and RA treatment history are summarized in Table 1. Patients in cohort A were older than those in cohort B [mean (SD) age 59.1 (13.2) versus 57.0 (13.9) years; p = 0.010]. The proportions of patients who were naïve to ts/bDMARDs for cohorts A and B were 48.1% and 60.9%, respectively; thus, the proportions of cohort A and B patients with exposure to ts/bDMARDs at any time before study entry were 51.9% and 39.1%, respectively. The proportions of cohort A and B patients who had previously received bDMARDs were 48.5% and 36.5%, respectively, and those for patients who had received tsDMARDs at any time before baseline were 6.1% and 6.4%, respectively. The proportions of cohort A patients who had received two or more tsDMARDs or bDMARDs at any time before baseline were 21.6% and 17.1%, respectively; the corresponding proportions of cohort B patients were 14.0 and 15.0%, respectively. Thus, the proportions of cohort A and B of patients who had at least two tsDMARDs or bDMARDs at any time before baseline were 38.7% and 29.0%, respectively. The main reason for discontinuing previous treatment with ts/bDMARDs in cohort A and cohort B was secondary loss of response (15.1% and 8.3%, respectively) followed by primary lack of response (6.3% and 5.0%, respectively) [Table 1]. Lastly, almost half of patients were receiving any type of oral glucocorticoids at the time of enrollment [cohort A: 42.8% (218/509); cohort B: 44.1% (249/565)].
The patients’ clinical characteristics, including swollen and tender joint counts, global and pain assessment scores, disease activity, and quality of life, were generally similar between cohorts (all p > 0.05; Table 2) with two exceptions. Patients in cohort A had a longer duration of RA from diagnosis to enrollment than those in cohort B [mean (SD) time 10.0 (9.1) versus 8.9 (9.6) years, respectively; p = 0.047; Table 1] and slightly worse physical functioning [mean (SD) HAQ-DI score 1.4 (0.7) versus 1.3 (0.7), respectively; p = 0.033; Table 2]. Patients in both cohorts had high disease activity [mean (SD) CDAI scores: cohort A: 24.0 (11.7) and cohort B: 23.8 (12.4); p = 0.799].
Most cohort A patients [450/509 (88.4%)] received baricitinib 4 mg, whereas the remaining patients [59/509 (11.6%)] received baricitinib 2 mg daily doses. In cohort B, most patients [499/565 (88.3%)] received bDMARDs; a comparatively low number of patients [66/565 (11.7%)] received tsDMARDs (other than baricitinib). Of patients treated with bDMARDs, most received etanercept [161/499 (32.3%)] and adalimumab [136/499 (27.3%)]; more than 80.0% of patients receiving each of these TNFis were prescribed biosimilars. The proportion of patients treated with monotherapy was higher in cohort A [baricitinib, 259/509 (50.9%)] than in cohort B [ts/bDMARDs, 176 (31.2%)].
Treatment Discontinuation
All treatment discontinuation data were descriptively analyzed, as discussed in the Statistical Analysis section. At 6 months, 483/509 (94.9%) cohort A and 539/565 (95.4%) cohort B patients were continuing in the study (Table 3). The proportions of patients who completed the 6-month visit within the defined period were 336/509 (66.0%) for cohort A and 371/565 (65.7%) for cohort B (Table 3); one patient in cohort B had sustained clinical response. The remaining patients either completed the 6-month visit out of the defined period [cohort A: 77/509 (15.1%); cohort B: 83/565 (14.7%); TNFi: 55/338 (16.3%); non-TNFi bDMARD: 21/161 (13.0%); tsDMARDs (other than baricitinib): 7/66 (10.6%)] or are continuing in the study but missed the 6-month visit [cohort A: 70/509 (13.8%); cohort B: 85/565 (15.1%); TNFi: 36/338 (10.7%); non-TNFi bDMARD: 33/161 (20.5%); tsDMARDs (other than baricitinib): 16/66 (24.2%)].
The treatment discontinuation rates for cohort A and B patients were 12.4% (63/509) and 16.5% (93/565), respectively (Table 3); the corresponding cumulative incidences (95% CI) of discontinuation were 16.5% (12.9–21.1) and 23.3% (19.1–28.2) of patients in each cohort, respectively (Fig. 1; Table 3). When baricitinib dose was considered, the cumulative incidence (95% CI) of discontinuation was 17.2% (13.3–22.2) with baricitinib 4 mg and 11.7% (5.0–26.1) with baricitinib 2 mg (Supplementary Table 1). In patients naïve to ts/bDMARDs at enrollment, the cumulative incidence (95% CI) of discontinuation was 10.0% (6.1–16.2), whereas in patients who had previously received ts/bDMARDs, it ranged from 16.5% (9.9–26.7) to 28.0% (17.8–42.3) depending on the number of previous treatments (Supplementary Table 1). The cumulative incidence (95% CI) of discontinuation was 13.2% (8.9–19.4) for patients who received baricitinib monotherapy and 20.0% (14.6–27.1) for patients who received baricitinib in combination with csDMARD therapy.
Cumulative incidence of discontinuation of RA treatment over 6 months in cohort A (baricitinib) and cohort B (ts/bDMARDs). Cumulative incidence developed with reverse Kaplan–Meier estimate. Patients with sustained clinical response were excluded from the primary outcome analysis. Patients who were lost to follow-up for any reason, including death, were censored at the last observation available. bDMARD biologic disease-modifying antirheumatic drug, CI confidence interval, RA rheumatoid arthritis, tsDMARD targeted synthetic disease-modifying antirheumatic drug
The reasons for discontinuation, as reported by the treating physicians, are presented in Table 3. Across cohorts, the main reason for discontinuation was primary nonresponse, being reported by 3.3% (17/336) and 5.8% (33/371) of cohort A and B patients, respectively.
Clinical Outcomes
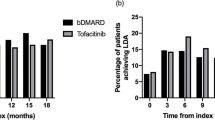
Treatment effectiveness analyses at baseline and 6 months included all patients with available CDAI data (cohort A: baseline 459, 6 months 250; cohort B: baseline 529, 6 months 276; Fig. 2). The mean (SD) CDAI score was 24.0 (11.7) at baseline and 10.0 (9.5) at 6 months for cohort A patients (Table 4); the respective CDAI scores for cohort B patients were 23.8 (12.4) and 11.8 (10.4). The mean (SD) changes from baseline to 6 months were −13.9 (12.5) and −11.8 (13.2) for cohort A and B patients, respectively. At 6 months, the proportions of cohort A and B patients achieving remission were 25.6% and 18.5%, respectively, whereas the corresponding proportions of patients achieving LDA were 36.8% and 37.3%, respectively (Fig. 2; Table 4). At 6 months, the proportions of cohort A patients achieving CDAI remission were 26.9% and 24.4% for those receiving baricitinib monotherapy or baricitinib in combination with csDMARDs, and the corresponding proportions of patients achieving LDA were 33.6% and 39.7% (Table 4); a similar picture emerged for cohort B patients receiving ts/bDMARDs monotherapy or ts/bDMARDs in combination with csDMARDs. During the observation period, the mean (SD) total cumulative prednisone equivalent dose of oral glucocorticoids taken was 1061.1 (954.2) mg for cohort A and 1093.9 (944.0) mg for cohort B.
Proportions of patients with CDAI remission and low, moderate, and high disease activity, at baseline and after 6 months of RA treatment with baricitinib (cohort A) and ts/bDMARDs (cohort B). Proportions of patients are calculated using as the denominator the number of patients with available CDAI data at baseline and 6 months and for each cohort. CDAI score categories were as follows: remission 0.0–2.8, low disease activity 2.9–10.0, moderate disease activity 10.1–22.0, and high disease activity 22.1–76.0. [23]. bDMARD biologic disease-modifying antirheumatic drug, CDAI Clinical Disease Activity Index, HDA high disease activity, LDA low disease activity, MDA moderate disease activity, RA rheumatoid arthritis, tsDMARD targeted synthetic disease-modifying antirheumatic drug
Regarding the PROs, improvements in the global and pain assessment scores, patients’ assessment of physical function, and quality of life were observed for both cohorts from baseline to 6 months (Table 4).
RA-related Health Care Resource Utilization
Across the five countries, the mean (SD) per patient number of visits (total and RA related) to primary care physicians, outpatient physicians, or emergency departments was similar between cohorts A and B, as was the mean (SD) per patient number and duration of hospitalization episodes (total and RA related; Supplementary Table 2). However, in France, the mean (SD) number of per patient visits (related to RA) to other health care professionals was 0.3 (1.5) and 4.1 (9.5) for cohort A and B patients, respectively; a similar picture emerged in Germany [cohort A: 1.3 (5.2) and cohort B: 2.6 (8.9)].
Discussion
The ongoing, prospective, observational, global 36-month RA-BE-REAL study primarily descriptively assesses the time until treatment discontinuation in patients with RA initiating treatment with baricitinib (cohort A) or another tsDMARD or any bDMARD (cohort B) for the first time at any point of their RA treatment algorithm in real-world settings. This report presents the 6-month interim analysis data from the European patients enrolled in RA-BE-REAL; upcoming planned RA-BE-REAL publications will report persistence with treatment over 2 years (primary study objective) and the treatment patterns over the entire study period.
Regarding the patients’ baseline demographic and clinical characteristics, it was noted that the baricitinib-treated patients were significantly (p = 0.010) older and had a significantly (p = 0.047) longer RA duration. The profile of patients initiating baricitinib, TNFis, or a bDMARD with another mode of action was assessed in a recent observational prospective study within the Swiss Clinical Quality Management register [19]. This study reported that the baricitinib-treated patients were older than those receiving TNFis and had a longer mean RA duration than the patients initiating TNFis or other mode of action bDMARDs (12, 9, and 11 years, respectively). The fact that baricitinib-treated patients in real-world settings have a slightly longer duration of RA than patients treated with another tsDMARD or any bDMARD may relate to its different mode of action. One could speculate that physicians chose baricitinib for patients who are not responsive to previous TNFis or other bDMARDs, and thus have a longer disease duration. This treatment approach is supported by the recent European Alliance of Associations for Rheumatology guidelines, which propose that if a ts/bDMARD has failed, treatment with another ts/bDMARD should be considered, whereas if a TNFi therapy has failed, patients may receive an agent with another mechanism of action or a second TNFi [2]. However, a network meta-analysis comparing cycling (i.e., using another agent with the same mode of action) versus switching (i.e., using agents with different modes of action) among RA treatments found that the latter approach was associated with improved clinical outcomes and lower withdrawal rates [28].
The main finding of this analysis was that the cumulative incidence of discontinuation at 6 months was 16.5% for patients initiating baricitinib and 23.3% for those initiating any other tsDMARD or any bDMARD. As 12.4% of baricitinib-treated patients had discontinued treatment over 6 months, the remaining 87.6% continued with baricitinib. This retention rate is aligned with rates reported by real-world studies conducted in Japan. The 6-month retention rate was 86.5% in a Japanese multicenter registry in 113 patients with RA, more than half of whom were previously treated with tsDMARDs [18], and 75.4% in a post-marketing surveillance study that included 4731 patients with RA treated with baricitinib [29].
In this analysis, primary nonresponse was the main reason for discontinuation, irrespective of treatment with baricitinib or any other tsDMARD or any bDMARD. This finding is consistent with reports from the USA [9], Japan [30, 31], and previous database searches [12], where the loss of efficacy or lack of effectiveness were the key reasons for discontinuations among patients receiving bDMARDs and/or tsDMARDs. Of note, in the current study, the proportion with primary nonresponse was 3.3% for baricitinib-treated patients and 5.8% for patients treated with another tsDMARD or any bDMARD.
Regarding the treatment patterns before baseline and during the observation period of RA-BE-REAL, two findings emerged. Before baseline, the proportions of patients with previous bDMARD exposure were 48.5% and 36.5% for baricitinib-treated patients and patients treated with another tsDMARD or any bDMARD, respectively. During the observation period, the proportions of patients receiving monotherapy were 50.9% and 31.2% for baricitinib-treated patients and patients treated with another tsDMARD or any bDMARD, respectively. This finding is aligned with the findings of the JAK-POT study, which evaluated the effectiveness of JAK inhibitors compared with bDMARDs in patients with RA initiating treatment with these agents in the usual care, using data from 17 international registries [32]. This study reported that JAK inhibitors were more often initiated as monotherapy than were bDMARDs. Moreover, consistent with the RA-BE-REAL findings, JAK-POT also reported that patients initiating JAK inhibitors had previously received more ts/bDMARDs than those initiating bDMARDs. However, JAK-POT reported that patients initiating JAK inhibitors had higher disease activity at baseline than those initiating bDMARDs, while the current study showed that patients had high disease activity in both cohorts.
Despite the between-cohort differences in the patients’ baseline characteristics mentioned earlier, the proportion of patients achieving remission at 6 months were 25.6% and 18.5% for baricitinib-treated patients and patients treated with another tsDMARD or any bDMARD, respectively; the corresponding proportions of patients achieving LDA over the same period were 36.8% and 37.3%, respectively. Finally, improvements in the PRO measures assessed and HCRU within each country were observed in both cohorts.
Several limitations need to be considered when interpreting the results of this interim analysis. The decision to discontinue study treatment was at the discretion of the treating physician and/or patient; thus, discontinuations could have been expedited because of the availability of many products for the treatment of RA. In this interim analysis we have used as “observed data” for the analysis of effectiveness (e.g., CDAI), as the 6-month observation period was insufficient to address all issues related to treatment and study discontinuation. The impact of comorbidities on treatment outcomes was not assessed. The possibility of patient selection bias cannot be ruled out, as the choice of treatment with baricitinib or another tsDMARD or any bDMARD was based on the investigator’s judgment. The intention of this manuscript was to present the results of the interim analyses descriptively, owing to immaturity of the data collected during the study follow-up of up to 6 months. In addition, as predefined in the statistical analysis plan, all the post-baseline analyses were descriptive; thus, the statistical significance of the between-cohort differences could not be inferred. However, due to this limitation, comparative analyses adjusted for potential confounders will be considered for future publications with more mature data.
Regarding HCRU, the data should be interpreted with caution given the limited observation period of 6 months. As expected with any real-world study focusing on time to discontinuation, adverse events were collected only within this context, whereas other adverse events were reported using the local pharmacovigilance programs of each country involved in the present study.
RA-BE-REAL is the first large-scale multinational study to generate real-world evidence on baricitinib. We believe that our analysis provides pertinent information regarding the incidence of and the reasons for early (within 6 months) treatment discontinuation of patients with RA initiating first-time baricitinib or any other tsDMARD or any bDMARD during routine clinical care in five large European countries. Furthermore, this analysis provides insight into the patient characteristics, the physicians’ prescribing habits, and key clinical outcomes in patients with RA initiating baricitinib or any other tsDMARD or any bDMARD. According to current knowledge, similar real-life data for baricitinib are sparse, and we anticipate that these findings will help inform the management of patients with RA.
Conclusions
This 6-month interim analysis of the European patients included in the RA-BE-REAL study showed that patients initiating baricitinib for the first time in their treatment algorithm were older and had a longer disease duration than patients initiating treatment with another tsDMARD or any bDMARD. Initial descriptive data on treatment discontinuation (including reasons for discontinuation), treatment patterns, effectiveness, PROs, and HCRU were generated. These data will be further enriched as RA-BE-REAL progresses.
References
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72. https://doi.org/10.1001/jama.2018.13103.
Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–99. https://doi.org/10.1136/annrheumdis-2019-216655.
Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924–39. https://doi.org/10.1002/acr.24596.
Smolen JS. Treat-to-target as an approach in inflammatory arthritis. Curr Opin Rheumatol. 2016;28(3):297–302. https://doi.org/10.1097/BOR.0000000000000284.
Carmona L, Gómez-Reino JJ, BIOBADASER Group. Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther. 2006;8(3):R72. https://doi.org/10.1186/ar1941.
Neovius M, Arkema EV, Olsson H, ARTIS Study Group, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015;74(2):354–60. https://doi.org/10.1136/annrheumdis-2013-204128.
Gottenberg JE, Morel J, Perrodeau E, et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ. 2019;364:l67. https://doi.org/10.1136/bmj.l67.
Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford). 2016;55(3):523–34. https://doi.org/10.1093/rheumatology/kev374.
Strand V, Miller P, Williams SA, et al. Discontinuation of biologic therapy in rheumatoid arthritis: analysis from the Corrona RA registry. Rheumatol Ther. 2017;4(2):489–502. https://doi.org/10.1007/s40744-017-0078-y.
Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–16. https://doi.org/10.1016/j.ejmech.2018.09.027.
Nikiphorou E, Negoescu A, Fitzpatrick JD, et al. Indispensable or intolerable? Methotrexate in patients with rheumatoid and psoriatic arthritis: a retrospective review of discontinuation rates from a large UK cohort. Clin Rheumatol. 2014;33(5):609–14. https://doi.org/10.1007/s10067-014-2546-x.
Fautrel B, Balsa A, Van Riel P, et al. Influence of route of administration/drug formulation and other factors on adherence to treatment in rheumatoid arthritis (pain related) and dyslipidemia (non-pain related). Curr Med Res Opin. 2017;33(7):1231–46. https://doi.org/10.1080/03007995.2017.1313209.
Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184(9):5298–307. https://doi.org/10.4049/jimmunol.0902819.
O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. https://doi.org/10.1146/annurev-med-051113-024537.
European Medicines Agency. Olumiant summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf. Accessed 12 Dec 2021.
Choy EHS, Miceli-Richard C, González-Gay MA, et al. The effect of JAK1/JAK2 inhibition in rheumatoid arthritis: efficacy and safety of baricitinib. Clin Exp Rheumatol. 2019;37(4):694–704.
Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–62. https://doi.org/10.1056/NEJMoa1608345.
Takahashi N, Asai S, Kobayakawa T, et al. Predictors for clinical effectiveness of baricitinib in rheumatoid arthritis patients in routine clinical practice: data from a Japanese multicenter registry. Sci Rep. 2020;10(1):21907. https://doi.org/10.1038/s41598-020-78925-8.
Gilbert B, Lauper K, Courvoisier D, et al. THU0203 real world effectiveness of baricitinib in The Swiss Rheumatoid Arthritis Register (SCQM-RA). 2020. https://ard.bmj.com/content/79/Suppl_1/325. Accessed 23 Sept 2021.
Gilbert B, Courvoisier D, Mongin D, et al. POS0668 real world effectiveness of baricitinib in the Swiss Rheumatoid Arthritis Register (SCQM-RA). Ann Rheum Dis. 2021;80:577–8.
Alten R, Burmester GR, Matucci-Cerinic M, et al. AB0261 A multinational, prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies (RA-BE-REAL)—study design and baseline characteristics. Ann Rheumatic Dis. 2021;80:1157.
World Medical Association. Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277(11):925–6.
Aletaha D, Smolen J. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–8.
Bruce B, Fries JF. The health assessment questionnaire (HAQ). Clin Exp Rheumatol. 2005;23(Suppl 39):S14-18.
Ramey DR, Fries JF, Singh G. The health assessment questionnaire 1995: status and review. In: Spiker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia: Lippincott-Raven; 1996. p. 227–37.
EuroQol Group. EQ-5D-5L User Guide. Version 2.1. 2015 [cited August 11, 2016]; https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Accessed 03 Sept 2021.
Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. https://doi.org/10.1016/0168-8510(96)00822-6.
Migliore A, Pompilio G, Integlia D, et al. Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: a Bayesian network meta-analysis. Ther Adv Musculoskelet Dis. 2021;13:1759720X211002682. https://doi.org/10.1177/1759720X211002682.
Takagi M, Atsumi T, Matsuno H, et al. Safety and effectiveness of baricitinib for rheumatoid arthritis in Japanese clinical practice: 24-week results of all-case post-marketing surveillance. Mod Rheumatol. 2022. https://doi.org/10.1093/mr/roac089.
Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther. 2019;21(1):91. https://doi.org/10.1186/s13075-019-1880-4 (Erratum in: Arthritis Res Ther 2019;21(1):114).
Ebina K, Hirano T, Maeda Y, et al. Drug retention of sarilumab, baricitinib, and tofacitinib in patients with rheumatoid arthritis: the ANSWER cohort study. Clin Rheumatol. 2021;40(7):2673–80. https://doi.org/10.1007/s10067-021-05609-7.
Lauper K, Mongin D, Bergstra SA, et al. OP0231 Comparative effectiveness of JAK-inhibitors, TNF-inhibitors, abatacept and IL-6 inhibitors in an international collaboration of registers of rheumatoid arthritis patients (The “JAK-POT” study). Ann Rheumatic Dis. 2020;79:146–7.
Acknowledgments
Funding
This study, including the journal’s Rapid Service Fee, was sponsored by Eli Lilly and Company, Indianapolis, IN, USA.
Medical Writing Assistance
The authors would like to acknowledge Ioannis Nikas and Karen Goa (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Author Contributions
All authors met the authorship criteria and contributed substantially to the conception and design (Rieke Alten, Pedro Lopez-Romero, Thorsten Holzkämper, Walid Fakhouri, Inmaculada de la Torre), acquisition of data (Rieke Alten), analysis and interpretation of data (Rieke Alten, Gerd R. Burmester, Marco Matucci-Cerinic, Jean H. Salmon, Pedro Lopez-Romero, Walid Fakhouri, Inmaculada de la Torre, Liliana Zaremba-Pechmann, Thorsten Holzkämper, Bruno Fautrel) and drafting of the manuscript (Thorsten Holzkämper, Inmaculada de la Torre). All authors revised the manuscript critically for important intellectual content, approved the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Prior Presentation
This manuscript is based on work that has been previously presented as an abstract at EULAR 2021 Virtual Congress (Rheumatology), 2–5 June 2021; a poster at Deutscher Rheumatologiekongress 2021—virtual, 15–18 September 2021; an abstract and poster at Virtual ISPOR Europe 2021, 30 November–3 December 2021; and an abstract and poster at ACR Convergence 2021—virtual, 5–9 November 2021.
Disclosures
Rieke Alten reports consulting fees, honoraria, and support for attending meetings and/or travel from Abbvie, BMS, Celltrion, Galapagos, Eli Lilly and Company, Novartis, Pfizer, Roche, and UCB. Gerd R. Burmester has received consulting fees and speaker honoraria from AbbVie, Eli Lilly, Galapagos, Gilead, and Pfizer. Marco Matucci-Cerinic has received consulting fees or honorarium from Actelion, Janssen, Inventiva, Bayer, Biogen, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Eli Lilly and Company, Pfizer, and Roche. Jean-Hugues Salmon has received consulting fees or honoraria from Eli Lilly and Company, Pfizer, AbbVie, and Galapagos. Bruno Fautrel has received research grants from AbbVie, Lilly, MSD and Pfizer, and consultancy fees from AbbVie, Amgen, Biogen, BMS, Celltrion, Fresenius Kabi, Galapagos, Gilead, Janssen, Lilly, Medac, MSD, Mylan, NORDIC Pharma, Novartis, Pfizer, Roche, Sandoz, Sanofi-Genzyme, SOBI, UCB. Pedro Lopez-Romero is a former employee of Eli Lilly and Company. Walid Fakhouri, Inmaculada de la Torre, Thorsten Holzkämper are employees of Eli Lilly and Company. Liliana Zaremba-Pechmann is an employee of HaaPACS, Schriesheim, Germany, who was contracted by Eli Lilly and Company to assist with this work.
Compliance with Ethics Guidelines
RA-BE-REAL was conducted in accordance with the ethical principles of the Helsinki Declaration of 1964 and the Good Clinical Practice guidelines and the applicable laws and regulations of the five European countries. All patients involved provided written informed consent, and the study protocol was approved by relevant ethics committees in each participating country. In Europe, these were the Health Research Authority and Health and Care Research Wales in England and Wales, the Ethics Committee of the University of Würzburg in Germany, the Est IV Ethics Committee in France, and the Ethics Committee of Bergamo in Italy.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the study being ongoing.
List of Investigators
The authors would like to acknowledge the investigators and participants in the RA-BE-REAL study. The RA-BE-REAL investigators are as follows: France: F Berenbaum, Pierre Marie Curie-Hopital Saint-Antoine; P Fardellone, CHU Nord; P Hilliquin, CH Sud-Francilien Hopital Gilles De Corbeil; C Leske, Centre Hospitalier CH de Cholet; C Saliot, Hospital La Source Orleans; M Nguyen, Hopital Cochin; T Schaeverbeke, ThierryHopital Pellegrin; M Soubrier, Centre Hospitalier Universitaire de Clermont-Ferrand; O Vittecoq, CHU de Rouen; B Combe, Hopital Lapeyronie—CH Montpellier; B Fautrel, CHU Pitie Salpetriere; H Marotte Centre Hospitalier Universitaire CHU de Saint-Etienne—Hopital Nord; E Shipley, Centre Hospitalier de Dax—Cote dArgent—Hopital de Jour Medico-chirurgical; J-H Salmon, Centre Hospitalier Universitaire CHU de Reims—Hopital Maison Blanche; M-C Boissier, Hopital Avicenne; RM Flipo, Hopital Roger Salengro; J-E Gottenberg, CHU Strasbourg–Hautepierre, Service de Rhumatologie; F Liote, C.H.U. Lariboisiere; A Basch, Infirmerie Protestante De Lyon; C Albert-Sabonnadiere, Centre Hospitalier de Cannes Simone Veil. Germany: R Alten, Schlossparkklinik—Akad. Lehrkrankenhaus Charite; M Feuchtenberger, MED/BAYERN OST GmbH, MVZ; K Krueger, Praxiszentrum St. Bonifatius; T Marycz, Praxis fuer Rheumatologie; R Max, Universitaetsklinikum Heidelberg; M Roser, Rheumatologische Schwerpunktpraxis; M Sieburg, Rheumatologische Facharztpraxis; P Sternad, Klinische Forschung im medizinischen Versorgungsalltag GbR; H-P Tony, Universitaetsklinikum Wuerzburg; B Koehler, Rheumazentrum Ratingen; S Zinke, Praxis fuer Innere Medizin und Rheumatologie; P Aries, Rheumatologie im Struenseehaus; G Burmester, Charit Universitaetsmedizin Berlin; J Henes, Universitaetsklinikum Tuebingen; P Kaestner, MVZ Ambulantes Rheumazentrum Erfurt; H Schwenke, Rheumatologisches MVZ Dresden GmbH; T Kupka, Rheumazentrum Kupka; G Lorenz, MVZ AGILOMED; I Schwarze, Dr. Med. Ilka Schwarze; B Heiling, Rheumapraxis Heidelberg; D Wernicke, Practice; P Roll, Rheumapraxis; W Ochs, Internistisch-rheumatologische Praxisgemeinschaft Bayreuth; M Schmitt-Haendle, Internistisch-rheumatologische Praxisgemeinschaft Bayreuth; H Heintz, Hamburger Rheuma Forschungszentrum I; H Kellner, Praxis Prof. Kellner; A-E Thiele, Practice; A Rossbach, Practice; C Eisterhues, Rheumatologische Praxis. Italy: RD Grembiale, Rheumatology Research Unit—Department of Health Sciences, University Magna Graeciaâ of Catanzaro; M Limonta, ASST Papa Giovanni XXIII; M Matucci Cerinic, Azienda Ospedaliera Universitaria di Careggi; G Valentini, AOU Università degli Studi della Campania Luigi Vanvitelli; E Quarta, Ospedale Galateo San cesareo; C Salvarani, Azienda Ospedaliero universitaria di Modena; FP Cantatore, Azienda Ospedaliero Universitaria Ospedali Riuniti; O Viapiana, UOC Reumatologia – Policlinico GB Rossi; S Dangelo, Ospedale San Carlo; A Afeltra, Univ Campus Biomedico; A De Cata, IRCCS Ospedale Casa Sollievo della Sofferenza; E Gremese, Policlinico Universitario A. Gemelli-IRCCS/Università Cattolica Sacro Cuore-Presidio Columbus; M Govoni, Azienda Ospedaliero- Universitaria S. Anna; CM Montecucco, IRCCS Policlinico san Matteo; C Bazzani, Spedali Civili di Brescia; R Perricone, Università di Roma Tor Vergata; G Guggino, Policlinico Universitario Paolo Giaccone; M Mosca, Azienda Ospedaliero-Universitaria Pisana; O Epis, ASST Grande Ospedale Metropolitano Niguarda; R Meliconi, Istituto Ortopedico Rizzoli; B Frediani, Azienda Ospedaliera Universitaria Senese. Spain: JJ Alegre Sancho, Hospital Universitario Doctor Peset; F Garcia Fructuoso, Hospital CIMA Sanitas; M Garcia Vivar, Hospital de Basurto; F Navarro Blasco, Hospital General Universitario de Elche; JC Rosas Gomez de Salazar, Hospital de la Marina Baja; J Sanchez Burson, Hospital Infantaluisa; P Vela Casasempere, Hospital General Universitario de Alicante; S Holgado Pérez, Hospital Universitario Germans Trias i Pujol; AM Millán, Hospital de la Santa Creu i Sant Pau; C Perez, Hospital del Mar; P Trenor, Hospital Clinico Universitario de Valencia. UK: T Barnes, Countess of Chester Hospital NHS Foundation Trust; Z Cole, Salisbury NHS Foundation Trust; J Maxwell, Royal Hallamshire Sheffield Teaching Hospital NHS Foundation Trust; B Hauser, Western General Hospital; A Salih, Warrington Hospital; T Sheeran, The Royal Wolverhampton NHS Trust Cannock Chase Hospital; J Taylor, Northampton General Hospital NHS Trust; E Chelliah-Gladston, Wrightington Hospital; Y Patel, Hull Royal Infirmary; B Kirkham, Guys Hospital; C-S Yee, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust; L Al-Sweedan, George Eliot Hospital; N Goodson, University Hospital of Aintree; M Bukhari, Royal Lancaster Infirmary University Hospitals of Morecambe Bay NHS Foundation Trust; R Reece, Freeman Hospital, Newcastle-Upon-Tyne NHS Foundation Trust Newcastle University; I Scott, Haywood Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pedro Lopez-Romero: Former Lilly employee.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alten, R., Burmester, G.R., Matucci-Cerinic, M. et al. The RA-BE-REAL Multinational, Prospective, Observational Study in Patients with Rheumatoid Arthritis Receiving Baricitinib, Targeted Synthetic, or Biologic Disease-Modifying Therapies: a 6-Month Interim Analysis. Rheumatol Ther 10, 73–93 (2023). https://doi.org/10.1007/s40744-022-00500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00500-6