Abstract
Osteoarthritis is the most common chronic joint disease affecting millions of people worldwide and a leading cause of pain and disability. Increasing incidence of obesity and aging of the population are two factors that suggest that the impact of osteoarthritis will further increase at the society level. Currently, there are no drugs available that can manage both structural damage to the joint or the associated pain. Increasing evidence supports the view that the Wnt signaling pathway plays an important role in this disease. The current concept, based on genetic and functional studies, indicates that tight regulation of Wnt signaling in cartilage is essential to keep the joint healthy. In this review, we discuss how this concept has evolved, provide insights into the regulation of Wnt signaling, in particular by Wnt modulators such as frizzled-related protein and DOT1-like histone lysine methyltransferase, and summarize preclinical evidence and molecular mechanisms of lorecivivint, the first Wnt antagonist in clinical development for osteoarthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Wnt signaling pathway plays an important role in osteoarthritis. |
Targeting of the Wnt signaling pathway is possible at different levels of the pathway’s activation cascade. |
The first Wnt inhibitor for treatment of osteoarthritis, lorecivivint, is entering phase III clinical trials. |
Introduction
Osteoarthritis is the most common chronic joint disease, affecting over 250 million people worldwide [1]. Over 80% of these patients report limitations with joint movement and over 25% are not capable of normally performing activities of daily life. Therefore, osteoarthritis is rightfully considered a serious disease with a very high socio-economic impact and a high burden on individuals. As aging and obesity feature prominently among risk factors for the development of disease and its progression, the impact of osteoarthritis on society is likely to further increase in the near future.
Currently, there is no cure for the disease and there are only limited treatment options. Standard approaches include physiotherapy and exercise programs combined with painkillers and anti-inflammatory drugs [2]. Local treatments of disease flares by intra-articular steroid or hyaluronic acid injections are part of common practice. Of note, strong painkillers have been associated with severe side effects, including increased mortality and rapidly progressive osteoarthritis in some patients [3, 4]. Currently, there are no so-called structure or disease-modifying treatments available and in huge groups of patients, in particular those with large joint involvement, prosthesis, or other types of joint surgeries may become the only viable solution. Prosthesis surgeries are a form of joint replacement rather than joint restoration approaches and the life-time of the implants may become too limited for the increased life expectancy of many patients, thus leading to re-do surgery, which is often challenging in particular in the very elderly.
The onset and course of osteoarthritis are not easily explained in a simple model. In fact, osteoarthritis is best considered as the outcome of different disease processes, representing a range of interactions between contributing mechanisms and factors depending on the characteristics of the individual patient. Risk factors include, among others, obesity, metabolic disorders, injury, altered anatomy and biomechanics of the joint, and inflammation [2]. With many different mechanisms contributing to the initiation as well as to the progression of disease, it is not surprising that the development of specific targeted therapies is a major challenge in the long process from bench to bedside, and that the clinical development of such strategies will need to focus on well-defined subpopulations in order to establish quality data on an eventual effect of the intervention. One molecular pathway of interest in this context is Wnt signaling [5]. In this review, we discuss how Wnt signaling was identified as a potential target for the treatment of osteoarthritis and we present the underlying mechanism of a Wnt signaling inhibitor that is currently progressing through clinical trials for the treatment of patients with knee osteoarthritis. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Earlier work from the authors was approved by the Ethical Committee for Clinical Research University Hospitals Leuven and the Ethical Committee for Animal Research KU Leuven.
Wnt Signaling: A Conserved Biological System with Potential for Great Diversity
Wnts are a family of at least 19 different secreted proteins in humans that affect a large array of biological processes. Originally defined as morphogens in organisms such as Drosophila melanogaster (fruit fly) and Xenopus laevis (tropical frog) or discovered as potential oncogenes in malignancies (for extensive review see [5,6,7]), our knowledge on their biological effects and impact has steadily grown over the years, albeit that many questions remain. The effects of individual Wnts have been difficult to study, not in the least because Wnts are poorly soluble and their isolation, specific identification as well as their production as recombinant molecules are highly challenging. A major reason for these research obstacles is found in the post-translational processing of the molecules in the cell before secretion. The Wnt ligands are linked with a lipid sidechain by an enzyme called porcupine before their secretion [7,8,9]. This lipid side chain strongly reduces the solubility of the Wnt ligand and its potential signaling range. We currently assume that Wnts have mostly autocrine and paracrine, rather than endocrine effects. Nevertheless, the affinity of Wnts for extracellular matrix or cell surface molecules like heparin-sulfated proteoglycans [10], and their binding to other secreted molecules such as those belonging to the secreted frizzled related protein (SFRP) family that can serve as molecular shuttles [11, 12], are factors that impact the signaling range of Wnts. Hence, following a biological principle from development, Wnts are molecules that typically build concentration gradients with an impact on adjacent cell behavior. As a consequence, targeting the Wnt signaling pathway for treatment of diseases may need to overcome a major challenge in targeting the cells and tissues where active signaling is contributing to pathology.
Wnt Signaling Plays a Key Role in Osteoarthritis
Early suggestions that Wnt signaling may play a role in cartilage biology and joint disease came from the progressive insights into how Wnt signaling plays a role in skeletal development [13]. The current view on these processes holds that low activity of the cascade in skeletal progenitor cells contributes to the process of cell condensation and commitment towards chondrogenic differentiation in bone development[6], whereas in later stages activation of Wnt signaling is essential in the progressive differentiation from proliferating chondrocytes towards hypertrophic cells thereby stimulating bone formation [13, 14]. In addition, Wnts have an anabolic impact on bone progenitor cells directly [13]. Together, these observations demonstrate how modulation of pathway activation controls the differentiation status of skeletal cells and how fine-tuning of Wnt activity is essential for normal development, as suggested by skeletal malformations associated with mutations in key pathway genes [15, 16].
A potential role for Wnt signaling in osteoarthritis emerged based on a series of different discoveries. First, in their search for genetic factors potentially associated with osteoarthritis, investigators found evidence that polymorphisms in the SFRP3 gene, which encodes Frizzled-related protein (FRZB), were associated with hip osteoarthritis [17]. FRZB was earlier identified as a secreted antagonist of the Wnt signaling cascade, as it is able to bind Wnt proteins and prevent Wnt–Wnt receptor interactions [11]. Interestingly, FRZB was first identified from a chondrogenic extract of articular cartilage and is expressed in the developing joint [18]. Functional analyses suggested that the identified osteoarthritis-associated variants in the SFRP3 gene affected the Wnt antagonizing properties of FRZB [17]. Interestingly, other investigators documented that expression levels of SFRP3 were rapidly downregulated upon in vitro cartilage injury [19, 20]. Our research group had developed Sfrp3 knockout mice to study the role of this molecule in skeletal development. However, primary analyses of this novel mouse strain did not demonstrate any obvious skeletal abnormalities. Nevertheless, when studying these animals postnatally, we demonstrated that the Sfrp3 knockout mice showed more severe osteoarthritis in different induced models, and that FRZB appeared to interact directly with cartilage matrix destructive enzymes such as matrix metalloproteinase-3 [21]. In addition, absence of FRZB also increased cortical bone thickness and anabolic responses upon in vivo loading in the long bones [21]. The data from this mouse model were thereby the first evidence that increased activation of Wnt signaling in the joint can contribute to the development of osteoarthritis, probably by multiple mechanisms.
FRZB and related SFRPs are soluble modulators of Wnt signaling. As they bind Wnt ligands directly, they antagonize interaction of the growth factors with cell-surface receptors. Key receptors for the Wnt cascade are those of the Frizzled family, molecules found in the cell membrane with an extra-cellular domain that can bind Wnts, and an intracellular tail that interacts with cytoplasmic proteins. The Wnt-Frizzled interaction attracts co-receptors lipoprotein-related protein 5 or 6 (LRP5/6) and triggers a downstream intracellular signaling cascade. A key molecule in the subsequent intracellular events is beta-catenin [5, 7] (Fig. 1). Beta-catenin is a multi-functional protein that is not only involved in the Wnt cascade but also in cell adhesion. In the absence of cell-surface induced Wnt signaling activation, beta-catenin is caught in a multi-protein complex and targeted for phosphorylation, ubiquitination and degradation by the proteasome. However, upon Wnt ligand-receptor complex interaction, the destruction complex is inactivated, allowing beta-catenin to accumulate within the cytoplasm and subsequently translocate towards the nucleus. Beta-catenin is not a transcription factor but does associate with transcription factors from the TCF/LEF family and binds in this way indirectly to its target genes, thereby inducing a specific transcriptional program within the cells [5, 7]. The beta-catenin-dependent Wnt pathway is also called the canonical Wnt pathway. Alternative cascades are less well studied but include intracellular calcium release, changes in cell polarity, and activation of stress kinases.
Simplified overview of canonical Wnt signaling pathway activation. Wnt ligands interact with Frizzled receptors and LRP5/6 co-receptors, which leads to the inactivation of the multi-protein beta-catenin destruction complex. This allows beta-catenin to accumulate within the cytoplasm and to translocate to the nucleus. Here, beta-catenin associates with transcription factors of the TCF/LEF family that bind to Wnt target gene promotors
A key role for beta-catenin in the maintenance of cartilage and joint health was subsequently confirmed by two studies with opposing strategies. First, cartilage-specific inactivation of beta-catenin was demonstrated to lead to articular cartilage destruction mainly by chondrocyte cell death [22], whereas overexpression of a constitutively active form of beta-catenin also resulted in osteoarthritis characterized by increased cartilage cell hypertrophy [23]. Together, these studies provided clear evidence that canonical Wnt signaling must be tightly regulated within the joint in order to maintain homeostasis and prevent joint disease such as osteoarthritis [24].
Fine-Tuning of Wnt Signaling is Essential for Joint Health
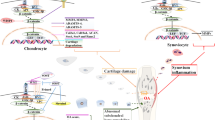
Since these original discoveries, a large body of evidence has been assembled that supports the view that controlling Wnt signaling is essential to maintain joint homeostasis and prevent disease. Strong activation of the canonical Wnt signaling pathway alters the molecular characteristics of the healthy articular chondrocyte, stimulates their further differentiation towards hypertrophic cells associated with the production of a calcified and therefore biomechanically inferior extracellular matrix. In contrast, in the superficial layer of the articular cartilage, Wnt16 production stimulates the synthesis and secretion of lubricin, a lubricant molecule that contributes to minimize friction between the opposing cartilage surface in the moving joint [25].
Osteoarthritis is a not a simple cartilage disease but a disorder that affects and involves all structures of the joint organ [26, 27] (Fig. 2). The primacy of cartilage versus subchondral bone changes in the pathogenesis has been extensively debated. The currently prevailing view suggests that different disease endotypes exist in which the initial changes may differ but ultimately lead to the complex process that leads to joint destruction and the associated clinical problems. Activation of canonical Wnt signaling appears to contribute towards increased subchondral bone remodeling and towards osteophyte formation, two pathological processes in the bone that are also associated with osteoarthritis development [24, 28]. Moreover, activation of Wnt signaling in the synovium contributes to inflammation and directly as well as indirectly towards progressive joint destruction [29].
Taking into account these myriad effects that activation of Wnt signaling can have on different cells and distinct tissues, and the intrinsic impact of such signals on a cell’s differentiation status, it should come as no surprise that Wnt signaling is tightly regulated at every level of the cascade. As highlighted above, the SFRPs are a family of secreted molecules that can bind and antagonize individual Wnt molecules. Other antagonists also exist, including sclerostin, a molecule typically produced by the bone-mechanosensing osteocytes, and that serves to maintain a tight regulation of the skeletal bone mass [16]. The dickkopf family (DKKs) are binding to the Wnt co-receptors LRP5 and 6, providing a non-ligand related mechanism to control the amplitude and extent of canonical pathway Wnt signaling activation [30]. The extent to which different individual Wnts trigger both types of cascades has not been well understood, but there is solid evidence of reciprocal interactions between canonical and non-canonical pathway activation [30].
Within the cell cytoplasm, the multi-protein destruction complex also contributes to fine tuning of signaling. Different phosphorylation sites on distinct members of the complex mediate their molecular function or turnover, thus providing specific targets to modulate this process. Small molecules inhibiting the activity of the destruction complex have been used in investigational settings to induce ligand-independent signaling pathway activation. The biology of the destruction complex remains an interesting field of study as there may be tissue-specific factors involved which would allow for the development of targeted therapies, for example for osteoarthritis.
The complexity of the signaling pathway and its involvement in a wide range of biological processes appears to be a daunting perspective for the development of therapies that have an impact on a disease such as osteoarthritis. In addition, therapies for an aging-associated disease that is slowly progressing may require sustained administration of drugs over a long time-period in a population of patients that will likely also show an increase in co-morbidities over time. Hence, safety of treatments is an important consideration [31]. We propose that there are at least two ways to overcome these challenges, either by searching for tissue-specific mechanisms of action or by developing highly tissue-specific targeting approaches for drug delivery.
Currently, multiple approaches can be considered (Fig. 3). Inhibition of porcupine will shut down Wnt secretion [32]. Antibodies or molecular mimics could be used to interfere with ligand–receptor interactions [33]. Such strategies may also be developed towards co-agonists such as the R-spondin family [34], molecules that amplify canonical signaling pathway activation [35]. Drugs can stabilize the destruction complex thereby stimulating beta-catenin breakdown [36]. Finally, intranuclear effects on gene transcription may confer the tissue specificity desired.
Potential therapeutic targets associated with activation of the Wnt signaling pathway. Inhibition of porcupine will stop Wnt ligand secretion. Wnt receptors, Wnt co-receptors, and accessory signals such as R-spondin LGR4/5 interactions can be inhibited. The destruction complex can be stabilized to increased beta-catenin degradation. Transcription complex formation can be disturbed by chemicals
DOT1-Like Histone Lysine Methyltransferase (DOT1L) is a Histone Methyltransferase that Modulates Wnt Signaling in Cartilage
We identified a key role for epigenetic enzyme DOT1L in the regulation of Wnt signaling in cartilage [37,38,39]. As for SFRP3, the first evidence for an eventual role of DOT1L in osteoarthritis came from genetic studies [39]. A genome-wide association study in a population cohort identified polymorphisms in the DOT1L gene to be associated with cartilage thickness in the hip. This variable was considered a proxy for the development of osteoarthritis, and subsequently the genetic association was also confirmed in an osteoarthritis patient versus control cohort. DOT1L is an enzyme that methylates lysine 79 on histone 3 (H3K79), resulting in a histone modification. Among histone methyltransferases, DOT1L is the major H3K79 methyl transferase [40, 41]. Histone modifications are one of the key epigenetic mechanisms to control gene expression. Within the cell nucleus, the DNA molecules are tightly wrapped around the histones for compaction and gene regulation. Modification of the histone protein tails by methylation or acetylation is a highly specific mechanism to affect the extent by which a particular stretch of DNA is wrapped around the histone and thereby to the availability of the DNA and genes for transcription factor binding and eventual transcription.
We subsequently demonstrated that DOT1L is a key factor in maintaining cartilage homeostasis and confers protection against the development of osteoarthritis [38]. Indeed, intra-articular injection of a small molecule that inhibits DOT1L activity reduced H3K79 methylation and triggered the development of osteoarthritis. Molecular analysis of human articular chondrocytes treated with DOT1L inhibitor also demonstrated gene expression similar to those seen in patients with osteoarthritis and with an enrichment for molecules of the Wnt signaling cascade. Further molecular analyses demonstrated that DOT1L is particularly important when Wnt signaling is activated, whereby it acts as a brake to limit the activation of the pathway. Effectively, Blockade of Wnt signaling by intra-articular injection of a Wnt inhibitor could rescue the effect of DOT1L inactivity in the mouse model. DOT1L is found in multi-protein complexes including beta-catenin, suggesting that it associated with the key mediator of Wnt signaling upon pathway activation. Within the nucleus, it also interacts with SIRT1, another epigenetic modulator of gene transcription. The different analyses performed allowed us to propose a model in which DOT1L activity is able to maintain cartilage homeostasis by restricting the activation of Wnt signaling, thus establishing an endogenous control mechanism [38]. We also found that levels of H3K79 methylation are reduced in patients with osteoarthritis, suggesting that the disease is associated with reduced DOT1L activity. A parallel approach in aging and trauma-triggered osteoarthritis mouse genetic models clearly confirmed that loss of DOT1L activity contributes to osteoarthritis [37]. Interestingly, at least some of these effects appeared to be cartilage-specific as the Wnt activation control mechanism documented in chondrocytes appeared to be largely absent in bone cells [38].
Lorecivivint is a Small Molecule Wnt Pathway Inhibitor
The important role of Wnt signaling in a number of diseases, including osteoarthritis and osteoporosis, has sparked interest from pharmaceutical and biotechnology companies to target the pathway for the treatment of these disorders. In a drug library screening, different Wnt modulators were identified of which lorecivivint (SM04690) was selected for studies on osteoarthritis [42]. Of note, the -vivint suffix definition has been attributed to the novel class of Wnt pathway inhibitors. Preclinical development highlighted the potential of lorecivivint to be used in osteoarthritis. In vitro experiments demonstrated that the addition of lorecivivint to differentiation cultures of human mesenchymal progenitor cells towards cartilage increased chondrogenesis, as demonstrated by increased extracellular matrix accumulation, including proteoglycans and collagens [42]. Further cartilage protection could be conferred by the inhibitory effects of lorecivivint on the expression of tissue destructive enzymes from the matrix metalloproteinase family [42]. Of note, the compound also demonstrated anti-inflammatory effects by reducing the expression of cytokines such as interleukin-1b, tumor necrosis factor and interleukin-6 in synovial fibroblasts and in peripheral blood mononuclear cells [43], two groups of cells that can play a role in the inflammatory reaction that is often seen in patients with osteoarthritis.
These promising results prompted the use of lorecivivint in in vivo models of osteoarthritis [42]. Remarkable, intra-articular injection resulted in sustained high levels of the molecule over several months, mostly in cartilage and to a far lesser extent in bone. Systemic levels of the drug were extremely low. Intra-articular injection also provided clear protection against osteoarthritis development in rats both in a surgical instability model [42] and in model characterized by inflammation and pain [43], thus paving the way for human trials.
Further in vitro work identified a unique mechanism of action for lorecivivint [43] (Fig. 4). The compound works as a kinase inhibitor and was demonstrated to mainly affect the activity of CDC-like kinase enzymes (CLKs) [43], in particular CLK2, in nanomolar concentrations, and dual-specificity tyrosine phosphorylation-regulated kinase enzymes (DYRK) [43], in particular DYRK1A, again in nanomolar concentrations. These molecules had not yet been clearly associated with Wnt signaling, thereby requiring further investigations. CLK2 plays an important role in the steps from DNA transcription towards protein translation. RNA that is the result of gene transcription requires further processing by splicing, for example to remove introns. Splicing is regulated by phosphorylation of pre-mRNA binding proteins such as the serine/arginine-rich splicing factors (SRSF) by CLKs. Inhibition of CLK2 affects this process and results in abnormal transcripts that may have dominant negative effects on the signaling cascade. Experimental data suggest that the effects on Wnt signaling transcription factors reduce the levels of Wnt target genes and simultaneously increase the levels of markers associated with the healthy chondrocyte profile such as aggrecan, collagen type 2, and DOT1L [43]. Moreover, negative modulation of CLK2 activity also appears to inhibit NF-kB signaling, a strong pro-inflammatory cascade.
Proposed dual mechanism of action of lorecivivint. Inhibition of CLK2 results in dysregulation of transcription and mRNA slicing leading to decreased expression of Wnt target genes and of inflammatory cytokines. Inhibition of DYRK1 reduces SIRT1 and FOXO1 phosphorylation, thereby inhibiting Wnt pathway activation and proinflammatory cascade while also having direct chondroprotective effects. Arrows indicate positive effects, closed lines (red) inhibitory effects
Kinase inhibitors rarely have total specificity, and this also applies to lorecivivint. Interestingly, in addition to inhibition of CLK2, effects on DYRK1A offers an interesting perspective on the mechanism of action. DYRK1A is associated with anti-inflammatory effects as it affects the JAK/STAT signaling pathway [43]. Moreover, DYRK1A also affects activity of transcription factor FOXO1 and of epigenetic modulator SIRT1 [43]. Inhibition of SIRT1 in a context of Wnt hyper-activation may contribute to cartilage homeostasis [38]. Positive regulation of FOXO1 stimulates cartilage homeostasis and lubricin production [44].
These preclinical data have been used to support the use of lorecivivint for the treatment of knee osteoarthritis in human clinical trials. Based on a phase 1 study with an excellent safety profile [45], phase II trials have recently been finished suggesting effectiveness on both signs and symptoms as well as structural damage in patients with knee osteoarthritis, in particular those without widespread pain [46, 47]. As a caveat, data are currently only available in meeting abstract format. In the first phase II trial, 455 patients with knee osteoarthritis were included and three different dosages (0.03, 0.07, and 0.23 mg) by single intra-articular injection were compared to placebo for up to 52 weeks, with the primary endpoint a change from baseline in the target knee in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain sub-score compared to placebo [47]. This primary endpoint was not met, but at all time-points clinically meaningful change from baseline in this parameter was seen. At 52 weeks, the 0.07 mg group showed significant improvement in the WOMAC pain and function score compared to placebo within a pre-specified group of unilateral knee osteoarthritis patients, an effect that was more pronounced in a pre-specified group of unilateral knee osteoarthritis patients without widespread pain. Hence, this first phase II trial suggested a target population and a potential optimal dosage. Of note, at 52 weeks, the 0.07 mg unilateral symptomatic and 0.07 mg unilateral symptomatic without widespread pain demonstrated a significant increase from baseline in medial joint space width, reflecting cartilage thickness, compared to placebo.
A phase IIb study over 24 weeks was then set up to refine outcome measures, target population and dosage, while further evaluating safety [46]. Single intra-articular injection over a wider dosage range (0.03, 0.07, 0.15, and 0.23 mg) was compared to placebo or sham-injection in 695 patients with clinically dominant unilateral knee osteoarthritis (high pain score in target knee, low pain score in contra-lateral knee). The study showed statistically significant differences between the 0.07 and 0.23 mg dosages for pain evaluated by Numeric Rating Scale (NRS), for WOMAC pain and physical function, as well as for patient global assessment. In both phase II trials, there were no apparent safety issues. The data convinced the company to continue its clinical development program and currently phase III trials are ongoing.
Conclusions
The Wnt signaling pathway is an important biological cascade in joint homeostasis and in joint diseases such as osteoarthritis. Wnt signaling is tightly regulated at all levels of the cascade thereby providing many opportunities for drug development. However, optimal strategies may require cell-specific and cell-directed targeting. DOT1L is an epigenetic modulator of the Wnt cascade that acts as a brake mechanism on signaling pathway activation thereby preventing cartilage damage. Lorecivivint is a Wnt inhibitor with a novel mechanism of action that is currently progressing in clinical trials and may have a double mechanism: differential splicing of target genes and anti-inflammatory effects. Extrapolation suggests that the former mechanism contributes to maintenance of the cartilage and joint structure, whereas the latter has symptomatic effects.
References
OARSI White Paper—osteoarthritis as a serious disease. https://www.oarsi.org/education/oarsi-resources/oarsi-white-paper-oa-serious-disease2016.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59.
Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969–82.
Katz JN. Tanezumab for painful osteoarthritis. JAMA. 2019;322(1):30–2.
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99.
Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol. 2008;19(5):434–43.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205.
Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134(18):3339–48.
Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10(24):3116–288.
Wang X, Cornelis FMF, Lories RJ, Monteagudo S. Exostosin-1 enhances canonical Wnt signaling activity during chondrogenic differentiation. Osteoarthr Cartil. 2019;27(11):1702–10.
Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88(6):757–66.
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–46.
Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317.
Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–87.
Boudin E, Fijalkowski I, Piters E, Van Hul W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum. 2013;43(2):220–40.
Balemans W, Van Hul W. The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology. 2007;148(6):2622–9.
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004;101(26):9757–62.
Hoang B, Moos M Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J Biol Chem. 1996;271(42):26131–7.
Dell'accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008;58(5):1410–21.
Dell'Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O'Dowd J, et al. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8(5):R139.
Lories RJ, Peeters J, Szlufcik K, Hespel P, Luyten FP. Deletion of frizzled-related protein reduces voluntary running exercise performance in mice. Osteoarthr Cartil. 2009;17(3):390–6.
Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58(7):2053–64.
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–211.
Monteagudo S, Lories RJ. Cushioning the cartilage: a canonical Wnt restricting matter. Nat Rev Rheumatol. 2017;13(11):670–81.
Nalesso G, Thomas BL, Sherwood JC, Yu J, Addimanda O, Eldridge SE, et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann Rheum Dis. 2017;76(1):218–26.
Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):43–9.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Funck-Brentano T, Bouaziz W, Marty C, Geoffroy V, Hay E, Cohen-Solal M. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014;66(11):3028–39.
van den Bosch MH, Blom AB, Schelbergen RF, Vogl T, Roth JP, Sloetjes AW, et al. Induction of canonical Wnt signaling by the alarmins S100A8/A9 in murine knee joints: implications for osteoarthritis. Arthritis Rheumatol. 2016;68(1):152–63.
Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C, et al. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193(3):551–64.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32.
Chen CW, Beyer C, Liu J, Maier C, Li C, Trinh-Minh T, et al. Pharmacological inhibition of porcupine induces regression of experimental skin fibrosis by targeting Wnt signalling. Ann Rheum Dis. 2017;76(4):773–8.
Raman S, Beilschmidt M, To M, Lin K, Lui F, Jmeian Y, et al. Structure-guided design fine-tunes pharmacokinetics, tolerability, and antitumor profile of multispecific frizzled antibodies. Proc Natl Acad Sci USA. 2019;116(14):6812–7.
Tan B, Shi X, Zhang J, Qin J, Zhang N, Ren H, et al. Inhibition of Rspo-Lgr4 facilitates checkpoint blockade therapy by switching macrophage polarization. Cancer Res. 2018;78(17):4929–42.
Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X, et al. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582(5):643–50.
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20.
Cornelis FMF, de Roover A, Storms L, Hens A, Lories RJ, Monteagudo S. Increased susceptibility to develop spontaneous and post-traumatic osteoarthritis in Dot1l-deficient mice. Osteoarthr Cartil. 2019;27(3):513–25.
Monteagudo S, Cornelis FMF, Aznar-Lopez C, Yibmantasiri P, Guns LA, Carmeliet P, et al. DOT1L safeguards cartilage homeostasis and protects against osteoarthritis. Nat Commun. 2017;8:15889.
Castano Betancourt MC, Cailotto F, Kerkhof HJ, Cornelis FM, Doherty SA, Hart DJ, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA. 2012;109(21):8218–23.
Min J, Feng Q, Li Z, Zhang Y, Xu RM. Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell. 2003;112(5):711–23.
Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol. 2002;12(12):1052–8.
Deshmukh V, Hu H, Barroga C, Bossard C, Kc S, Dellamary L, et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthris Cart. 2018;26(1):18–27.
Deshmukh V, O'Green AL, Bossard C, Seo T, Lamangan L, Ibanez M, et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthr Cartil. 2019;27(9):1347–60.
Matsuzaki T, Alvarez-Garcia O, Mokuda S, Nagira K, Olmer M, Gamini R, et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aan0746.
Yazici Y, McAlindon TE, Fleischmann R, Gibofsky A, Lane NE, Kivitz AJ, et al. A novel Wnt pathway inhibitor, SM04690, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24-week, randomized, controlled, phase 1 study. Osteoarthr Cart. 2017;25(10):1598–606.
Yazici Y, McAlindon TE, Gibofsky A, Lane NE, Latterman C, Skrepnik N, et al. Efficacy and safety from a phase 2b trial of sm04690, a novel, intra-articular, Wnt pathway inhibitor for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2019;27:S503-S.
Yazici Y, McAlindon TE, Gibofsky A, Lane NE, Clauw DJ, Jones MH, et al. Results from a 52-week randomized, double-blind, placebo-controlled, phase 2 study of a novel, intra-articular Wnt pathway inhibitor (sm04690) for the treatment of knee osteoarthritis. Osteoarthr Cartil. 2018;26:S293–S294294.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Leuven Research and Development, the technology transfer office of KU Leuven has received consultancy and speaker’s fees, and research grants on behalf of Rik Lories from AbbVie, Boehringer-Ingelheim, Celgene, Eli-Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Samumed, and UCB. Academic research in the Lories & Monteagudo laboratory is supported by FWO Vlaanderen (Flanders Research Foundation), KU Leuven, the Excellence of Science initiative of the Federal Government, the EU H2020 and IMI2 program, Foreum and the VIB-Grand Challenges Program.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Earlier work from the authors was approved by the Ethical Committee for Clinical Research University Hospitals Leuven and the Ethical Committee for Animal Research KU Leuven.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12038913.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lories, R.J., Monteagudo, S. Review Article: Is Wnt Signaling an Attractive Target for the Treatment of Osteoarthritis?. Rheumatol Ther 7, 259–270 (2020). https://doi.org/10.1007/s40744-020-00205-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-020-00205-8