Abstract
Introduction
Tocilizumab (TCZ) monotherapy has been proven as an effective treatment for rheumatoid arthritis (RA) in clinical trials. However, there are limited data available regarding the effectiveness of TCZ monotherapy in real-world clinical settings in the United States. The objective of this study was to evaluate the impact of TCZ monotherapy on disease activity and patient-reported outcomes (PROs) in a US-based observational cohort of patients with RA seen in routine clinical practice.
Methods
Eligible patients had active RA, no prior use of TCZ, and initiated TCZ as monotherapy. Changes in disease activity and PROs were assessed 1 year after TCZ initiation for the overall cohort and stratified by number of prior tumor necrosis factor inhibitors (TNFis; 0, 1, or ≥2). Primary outcomes were change in Clinical Disease Activity Index (CDAI); change in patient global disease activity, pain, fatigue; and the proportions of patients with improvement in modified Health Assessment Questionnaire (mHAQ), morning stiffness, and EQ-5D.
Results
Of 255 eligible TCZ monotherapy initiators, 9.4% were TNFi naive, 36.5% had one prior TNFi, and 54.1% had ≥2 prior TNFis. Clinical and PRO measures indicated that patients were substantially impacted by their disease at baseline. The median decrease in CDAI from baseline to 1 year was 9.8 and median patient global and pain scores improved by 10 mm, indicative of clinically meaningful improvement; the median fatigue score improved by 5 mm. Approximately 26% of patients reported clinically meaningful improvement in mHAQ, 54% experienced improvement in morning stiffness, and 20% to 36% experienced improvement in EQ-5D domains (walking, self-care, usual activities, pain/discomfort, and anxiety/depression). Improvements were similar across TNFi groups.
Conclusions
Patients with active, refractory RA who initiated TCZ monotherapy experienced improvements in both composite disease activity scores and PROs at 1 year, regardless of prior TNFi exposure.
Funding
Corrona, LLC and Genentech.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many patients with rheumatoid arthritis (RA) experience diminished health-related quality of life (HRQOL), as well as increased disability and RA-related comorbidities [1, 2]. The goal of treatment in patients with RA is to reduce disease activity and improve patients’ HRQOL. In addition to traditional measures of clinical disease activity, patient-reported outcomes (PROs) are important measures in evaluating response to therapy in patients with RA [3,4,5]. PROs take into account HRQOL measures that impact patients’ experiences and activities of daily living, including pain, fatigue, discomfort, emotional health, and the ability to do usual physical activities.
Tocilizumab (TCZ) is a monoclonal antibody that blocks the interleukin-6 receptor and is approved for the treatment of patients with moderate-to-severe RA who have had an inadequate response to ≥1 disease modifying antirheumatic drug (DMARD). TCZ can be administered as monotherapy or in conjunction with conventional synthetic DMARDs (csDMARDs), such as methotrexate (MTX). Clinical studies have demonstrated that TCZ has similar efficacy when administered as monotherapy or in combination with csDMARDs [6, 7]. In the USA, patients initiating TCZ often have prior tumor necrosis factor inhibitor (TNFi) exposure. It is estimated that up to 40% of patients treated with a TNFi have an inadequate response [8,9,10]. Because TCZ is used later in the line of therapy, patients initiating TCZ tend to be older, with longer disease duration, and have more disability and impairment than patients initiating TNFis [11].
TCZ monotherapy has been proven to be effective for the improvement of RA disease activity in multiple clinical trials [6, 12, 13], and may be an effective treatment option for patients with RA who cannot tolerate or prefer not to use MTX. However, there are limited data regarding the impact of TCZ monotherapy on PROs. Additionally, clinical trial studies of treatments for RA may not be predictive of typical patient outcomes. Randomized clinical trials usually include select patient populations with high RA disease activity and may have strict requirements with regard to prior RA therapy exposure. Thus, findings from clinical trials may be less generalizable to clinical practice than data from observational studies conducted in real-world settings [14, 15]. The objective of this study was to evaluate the impact of TCZ monotherapy on clinical outcomes and PROs in a large, US-based, observational cohort of patients with RA (Corrona), both overall and stratified by prior TNFi exposure.
Methods
Study Setting
The Corrona registry has been previously described in detail [16]. Briefly, the Corrona RA registry (NCT01402661) is an independent, prospective observational cohort that collects longitudinal real-world data from patients and their treating rheumatologists [17, 18]. The registry includes patients recruited by 656 participating rheumatologists from 169 private and academic practice sites across 40 states in the United States. As of June 30, 2016, data on 43,099 patients with RA have been collected. Corrona’s database currently includes information from 326,613 patient visits and 145,526.5 patient-years of follow-up observation, with a mean duration of patient follow-up of 4.13 years (median, 3.33 years).
The study was conducted according to the current (2013) version of the Declaration of Helsinki. Ethics approvals for this study were obtained from New England Independent Review Board (IRB# 120160610) for private practice sites and local institutional review boards of participating academic sites.
Study Analysis Population
Eligible participants were patients with RA in the Corrona registry who were TCZ naive and initiated TCZ as monotherapy between January 1, 2010 and March 31, 2015. Patients in Clinical Disease Activity Index (CDAI) remission (CDAI ≤ 2.8) were excluded. Patients must have had follow-up data available after TCZ initiation with CDAI measurements at baseline (around the time of TCZ initiation) and 1 year (9–15 months) after treatment. Patients were included in the analyses regardless of the addition of a csDMARD, switch to another biologic, or discontinuation of TCZ without switching prior to the 1-year follow-up visit.
For this study, data from Corrona were collected from physician and patient questionnaires completed during routine clinical encounters that occurred over the 1-year study period. Data recorded at the time of clinical encounter included use of csDMARDs and biologics; 28-joint tender counts (TJC) and 28-joint swollen counts (SJC); CDAI; 28-joint Disease Activity Score based on erythrocyte sedimentation rate (DAS28-ESR); patient assessments of global disease activity, pain, and fatigue; modified Health Assessment Questionnaire (mHAQ); and EQ-5D.
Assessments and Outcomes
Disease activity and PROs at 1 year were assessed using clinically validated measures. Disease activity was evaluated using the median change from baseline in CDAI and the level of disease activity achieved at 12 months [19, 20]. As a sensitivity analysis, we evaluated the median change from baseline in DAS28-ESR and the level of disease activity achieved at 12 months in the subset of patients who had available DAS28-ESR values.
PROs included patient global assessment of disease activity, pain, and fatigue (0–100 mm on a visual analog scale [VAS]) [21,22,23,24]; meaningful improvement in mHAQ, defined as an improvement >0.25 on a scale of 0 (without any difficulty) to 3 (unable to perform) [25]; improvement in duration of morning stiffness [26]; and EQ-5D, which records patient-reported HRQOL across five domains (walking, self-care, usual activities, pain/discomfort, and anxiety/depression), the results of which can be examined using a summary index (0–1) or by individual evaluation of each domain [27]. Each EQ-5D domain is divided into three levels of severity: severe disability, moderate disability, or no disability. Improvement in individual EQ-5D domains was defined as patients improving from moderate disability to no disability or from severe disability to moderate or no disability.
Statistical Analysis
Data on demographics, insurance status, comorbid conditions, RA disease characteristics, and RA medications were available for ≥99% of patients. With respect to outcomes, disease activity based on the CDAI, and PROs such as patient global assessment, pain, and mHAQ were available in >98% of patients. Fatigue and the EQ-5D were added to the registry patient questionnaire toward the end of 2010; thus, patients who initiated TCZ earlier in 2010 did not have baseline ascertainment of these two PROs and were excluded from analyses examining change in these PROs over the study period. For all analyses, we excluded patients who had missing data (baseline and/or follow-up) for the outcome of interest.
Clinical outcomes and changes from baseline in PROs were assessed at 1 year (±3 months) and stratified by prior TNFi use (0, 1, or ≥2 prior TNFis). For patients with two visits within the 9–15-month time window, the visit closest to 12 months was used for the 1-year visit. For patients who discontinued TCZ within 12 months and subsequently initiated another biologic (defined as “switching”), CDAI was assessed using the last observation (before the switch) carried forward. For patients who discontinued TCZ within 12 months without switching to another biologic, disease activity at the 12-month visit was used for all analyses. Comparisons between patients who had received one or ≥2 prior TNFi were analyzed using χ2 tests, Fisher exact tests, t tests, or Wilcoxon-Mann–Whitney tests, as appropriate. Additional subgroup analyses were conducted to examine outcomes in TCZ monotherapy initiators who remained on monotherapy for 12 months.
Results
Patient Demographics and Clinical Characteristics
A total of 500 patients with RA initiated TCZ as monotherapy between January 1, 2010 and March 31, 2015 (Fig. 1). Among patients excluded from the analysis, 242 were excluded due to lack of follow-up information and/or missing CDAI information at baseline or 12 months; this group included patients who initiated TCZ monotherapy late in the study window and thus did not have a follow-up visit during the study period, patients who initiated TCZ monotherapy between Corrona visits and thus did not have a baseline CDAI assessment, and patients who did not have a follow-up visit within the 9–15-month window of interest. Three additional patients were excluded due to CDAI ≤ 2.8 at baseline. To address any potential bias in the patient selection process, we evaluated the demographics and clinical characteristics of patients who were excluded from the analysis. Patients who were excluded from the analysis were similar with respect to age, sex, RA disease duration, and baseline CDAI compared with those who were included in the analysis.
Patient demographics and baseline disease characteristics are described in Table 1. Of the 255 eligible TCZ monotherapy initiators who were included in the analysis, 9.4% were TNFi naive, 36.5% had received one prior TNFi, and 54.1% had received ≥2 prior TNFis. Most patients were female (80.8%) and the overall median (interquartile range [IQR]) age was 61 (49–69) years. The majority of patients (48.2%) were in ACR functional class II (Table S1). Patients who had received ≥2 prior TNFis were younger than patients who had received one prior TNFi (median [IQR] age, 58 [48–66] vs. 65 [55–73] years; P = 0.0002). A significantly higher proportion of patients with ≥2 prior TNFis were white and had a history of cardiovascular disease compared with patients with one prior TNFi. The majority of patients (63.5%) in the total study population had received ≥1 prior non-TNFi biologic. Of the patients who had received ≥2 prior TNFis, 50.0% had also received one prior non-TNFi and 18.8% had received ≥2 prior non-TNFis.
Baseline Clinical Disease Activity and PROs
Baseline disease activity and PROs are described in Table 1. The median (IQR) CDAI score among all patients was 24.0 (16.7–34.0); 8.2% of patients had low disease activity (LDA; CDAI >2.8 to ≤10), 25.7% had moderate disease activity (MDA; CDAI >10 to ≤22), and 56.1% had high disease activity (HDA; CDAI > 22) (Table S1). Patients had a median (IQR) SJC of 6 (2–10) and TJC of 8 (3–13). Disease activity measures were similar between patients previously treated with one vs. ≥2 prior TNFis, with baseline median (IQR) CDAI scores of 22.2 (14.0–30.0) and 25.2 (19.0–34.1), respectively (P = 0.153). Of the patients who had received one prior TNFi, 7.5% had LDA, 40.9% had MDA, and 51.6% had HDA at baseline, while 8.0%, 31.1%, and 60.9% of patients who had received ≥2 prior TNFis had LDA, MDA, or HDA, respectively (Table S1). Patients with one prior TNFi had a median (IQR) SJC of 6 (3–10) and TJC of 7 (2–12), and those with ≥2 prior TNFis had a median SJC of 6 (2–9) and TJC of 8 (4–14).
The overall baseline median (IQR) scores for patient global assessment, pain, and fatigue were 55.0 (40.0–75.0), 60.0 (40.0–76.0), and 60.0 (33.0–80.0) mm, respectively. The baseline median (IQR) mHAQ score among all patients was 0.6 (0.3–1.0), and patients reported a median (IQR) of 1 (0.5–2.5) h of morning stiffness. The baseline median (IQR) EQ-5D score of the total study population was 0.69 (0.59–0.78), with the majority of patients reporting at least some problems in walking (73.0%), self-care (81.5%), usual activities (94.0%), and pain and discomfort (94.0%); additionally, 43.7% of patients reported at least some problems with anxiety or depression (Tables 1 and S2).
Patients who had received one or ≥2 prior TNFis had similar baseline median (IQR) scores in patient global assessment (50.0 [30.0–70.0] vs. 60.0 [40.0–75.0] mm; P = 0.212), pain (60.0 [35.0–76.0] vs. 65.0 [40.0–80.0] mm; P = 0.466), fatigue (50.0 [23.5–80.0] vs. 65.0 [45.0–80.0] mm; P = 0.091), and mHAQ (0.5 [0.3–1.1] vs. 0.6 [0.3–0.9]; P = 0.761) (Table 1). Patients in both groups experienced a median duration of morning stiffness of 1 (IQR: one prior TNFi, 0.59–0.78; ≥2 prior TNFis, 0.59–0.78) h and had a baseline median EQ-5D score of 0.69 (IQR: one prior TNFi, 0.5–2.0; ≥2 prior TNFis, 0.5–3.00), with similar proportions of patients in the groups receiving one or ≥2 prior TNFis reporting at least some problems with walking (72.6% vs. 72.0%), self-care (50.0% vs. 51.8%), usual activities (80.8% vs. 80.8%), pain/discomfort (94.5% vs. 94.6%), and anxiety/depression (41.1% vs. 44.8%) (Tables 1 and S2).
Disease Activity 1 Year After Initiation of TCZ Monotherapy
After 1 year (median [IQR], 12 [11–13] months) of follow-up, 109 patients (42.7%) were still receiving TCZ as monotherapy, 66 (25.9%) had switched to another biologic, 48 (18.8%) were receiving TCZ in combination with a csDMARD, and 32 (12.5%) discontinued TCZ without switching (Table S3). The primary reason for discontinuation or switching was lack of efficacy (62.9%); 19.4% of patients discontinued or switched due to issues with safety and 4.8% for a combination of efficacy and safety reasons. The rates of switching to another biologic, adding a csDMARD, and discontinuation were similar across TNFi groups (Table S3).
The overall median (IQR) decrease in CDAI from baseline to 1 year was 9.8 (8.2–11.4) (Fig. 2a). At 1 year, 11.4% of patients were in remission, 31.0% had LDA, 31.4% had MDA, and 26.7% had HDA (Fig. 2b). Improvement in CDAI and achievement of LDA or remission were similar across the TNFi groups (Fig. 2). The median (IQR) change from baseline in CDAI was 10.6 (7.7–13.6) in patients who had received one prior TNFi and 9.6 (7.6–11.6) for those who had received ≥2 prior TNFis (P = 0.547). Approximately 11% of patients in both groups were in remission at 1 year; in the group with one prior TNFi, 34.4%, 32.2%, and 22.6% of patients had LDA, MDA, and HDA, respectively, while in the group with ≥2 prior TNFis, 28.3%, 32.6%, and 28.3% of patients had LDA, MDA, and HDA, respectively. Similar results were seen in the population of patients with available DAS28-ESR (Figure S1).
Improvement in clinical outcomes 1 year after initiation of TCZ monotherapy, overall and stratified by prior TNFi experience. a Median change from baseline in CDAI. b Shifts in CDAI category from baseline to 1 year. Remission was defined as CDAI ≤2.8; low disease activity as CDAI >2.8 to ≤10; moderate disease activity as CDAI >10 to ≤22; and high disease activity as CDAI >22. CDAI Clinical Disease Activity Index, TCZ tocilizumab, TNFi tumor necrosis factor inhibitor
Among those patients who persisted on TCZ monotherapy over 12 months (n = 109), the median (IQR) decrease in CDAI was 12.6 (3.3–19.0). At 12 months, 14.7% of patients were in remission, 33.9% had achieved LDA, 33.9% had MDA, and 17.4% had HDA.
PROs 1 Year After Initiation of TCZ Monotherapy
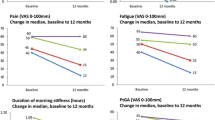
Improvement from baseline was observed in all PRO measures. Overall, the median (IQR) improvement from baseline in both patient global assessment and pain was 10 (−5 to 30) mm; median (IQR) improvement in fatigue was 5 (−10 to 23) mm (Fig. 3a; Table S4). Patients had a median percent improvement from baseline of 23.1% in patient global assessment, 22.2% in pain, and 10.8% in fatigue at 12 months. In the total study population, 26.1% of patients experienced clinically meaningful improvement in mHAQ 1 year after initiating TCZ monotherapy (Fig. 3b). Approximately half (54.0%) of patients in the total study population had improvement in morning stiffness, with 19.4% of patients achieving a reduction of >60 min and 34.6% achieving a reduction of 1 to 60 min (Fig. 3c). Patients also experienced improvement in all EQ-5D domains, with 20.8% of patients reporting at least some improvement in walking, 36.2% in self-care, 26.7% in usual activities, 21.3% in pain/discomfort, and 33.0% in anxiety/depression (Fig. 4).
Improvement in PROs 1 year after initiation of TCZ monotherapy overall and stratified by prior TNFi experience. a Median improvement from baseline in patient global assessment, pain, and fatigue scores. The full data set is described in Table S3. b Proportion of patients who experienced clinically meaningful improvement in mHAQ, defined as an improvement >0.25 [23]. Data are the percentage of patients reporting improvement among those patients who reported difficulty in each measure at baseline. c Proportion of patients who experienced reduction in duration of morning stiffness. mHAQ modified Health Assessment Questionnaire, TCZ tocilizumab, PROs patient-reported outcomes, TNFi tumor necrosis factor inhibitor
Improvement in EQ-5D categories at 1 year among TCZ initiators, overall and stratified by prior TNFi experience. aPercentage of patients reporting improvement among those patients who reported difficulty in each measure at baseline. bImprovement in the EQ-5D domains was defined as either an improvement from moderate to no disability, or from severe disability to moderate or no disability. * P < 0.05. The P value applies to the comparison between patients with one prior TNFi vs. those with ≥2 prior TNFis. TCZ tocilizumab, TNFi tumor necrosis factor inhibitor
Patients who remained on TCZ monotherapy over the 12-month study period had median improvement from baseline of 15.0 (0–33.0) mm for patient global assessment, 12.0 (0–34.0) mm for pain, and 5.0 (−9.0 to 25.0) mm for fatigue. Furthermore, 53.3% of these patients had a reduction in morning stiffness. Patients in this group also experienced improvement in the EQ-5D domains, with 20.6% having an improvement in walking, 36.2% in self-care, 32.9% in usual activities, 24.4% in pain and discomfort, and 28.6% in anxiety/depression.
Improvements in patient global assessment, fatigue, mHAQ, and morning stiffness were similar across TNFi groups (Fig. 3 and Table S4). However, patients with ≥2 prior TNFis had significantly greater improvement in median (IQR) pain score than patients with one prior TNFi (15 [0–35] vs. 8 [−5 to 25] mm; P = 0.050). A significantly higher proportion of patients with one prior TNFi experienced improvement in the EQ-5D domains of walking and usual activities than did patients with ≥2 prior TNFis (Fig. 4; walking, 35.3 vs. 10.8%; P = 0.001; usual activities, 35.6 vs. 18.9%; P = 0.022).
Discussion
In this study, patients with active, refractory RA who had an inadequate response to prior csDMARDs and/or TNFis and newly initiated TCZ monotherapy experienced substantial improvement in clinical disease activity and HRQOL 1 year after TCZ initiation, regardless of prior TNFi exposure. At baseline, the majority of patients were in high disease activity and reported substantial impacts of their disease on HRQOL. As indicated by the change from baseline in median CDAI (9.8), >50% of patients experienced clinically important improvement in disease activity (minimal clinically important difference [MCID] ≥ 6) at 1 year [20]. Furthermore, >40% of patients were in remission or had LDA as assessed by CDAI. Over half of patients reported clinically meaningful improvement in patient global assessment and pain (MCID ≥ 10 mm), as indicated by median improvement from baseline in both PROs of 10 mm. Approximately 26% of patients experienced clinically meaningful improvement in mHAQ (MCID > 0.25), and the majority of patients (54%) experienced at least some improvement in morning stiffness. Additionally, 20% to 36% of patients experienced improvement in EQ-5D domains (walking, self-care, usual activities, pain/discomfort, and anxiety/depression). Improvements in disease activity and PROs were mostly similar across TNFi groups; however, a significantly higher proportion of patients who had received one prior TNFi experienced improvement in walking and usual activities of daily living than did patients who had received ≥2 prior TNFis, while patients with ≥2 prior TNFis had significantly greater improvement in pain than those with one prior TNFi.
Clinical trials have demonstrated the efficacy of TCZ in the improvement of both disease activity and HRQOL. In ACT-RAY, which evaluated the efficacy and safety of TCZ monotherapy compared with that of TCZ + MTX in patients with an inadequate response to MTX, 15.6% of patients treated with TCZ monotherapy achieved CDAI remission and patients experienced significant improvements from baseline in mean patient global assessment (40.9) and pain (38.4) at 1 year [28]. The improvements observed in our study are comparable but slightly lower than those observed in ACT-RAY, with 11% of patients achieving CDAI remission and mean improvements from baseline in patient global assessment and pain of 12.0 and 13.9, respectively. Of note, patients in ACT-RAY had higher disease activity at baseline, as indicated by higher mean SJC and TJC, than patients in our study (15.3 vs. 6.0 and 26.6 vs. 8.0, respectively). Because baseline disease activity levels in real-world settings tend to be lower than those reported in randomized trials, there is the potential for a “floor effect” of clinical response; that is, it is more of a numerical challenge to improve a disease activity metric if a patient has a lower disease burden at baseline compared with those in clinical trials. Additionally, the majority of patients in our study had prior TNFi experience whereas those in ACT-RAY were biologic naive. Due to the real-world setting, it is possible that the patient population in our study was more refractory to biologic therapy at initiation of TCZ compared with those in ACT-RAY.
There are few large, observational, real-world studies evaluating the effectiveness of TCZ monotherapy in improvement of both disease activity measures and PROs. In an observational cohort study of the Tsurumai Biologics Communication Registry in Japan, the proportion of patients with DAS28-ESR <2.6 increased from 2% at baseline to 37% at 1 year after initiating TCZ monotherapy and patients experienced a mean improvement from baseline in patient global assessment of approximately 15 mm [29]. Similarly, the proportion of patients with DAS28-ESR <2.6 in our study increased from 7% at baseline to 39% at 1 year after initiating TCZ monotherapy, and patients experienced a median improvement from baseline in patient global assessment of 10 mm. Importantly, baseline disease characteristics were similar between patients from the Tsurumai Biologics Communication Registry and patients in this study, including disease duration (8.7 vs. 10.0 years), SJC (7.0 vs. 6.0), and TJC (8.7 vs. 8.0) [29].
These data suggest that in clinical practice, initiation of TCZ monotherapy at any stage of the RA treatment regimen may improve clinical outcomes and HRQOL. In this population of patients with established and severe RA, patients demonstrated substantial and clinically meaningful improvements in disease activity and HRQOL with TCZ monotherapy, regardless of prior TNFi exposure. At baseline, the majority of patients reported problems with walking, self-care, usual activities, pain/discomfort, and anxiety/depression. One year after initiating TCZ monotherapy, 20% to 36% of patients experienced at least some improvements in these HRQOL measures, even those with prior exposure to multiple TNFis. Of note, patients who received TCZ earlier in the line of treatment (one prior TNFi) had better response with respect to improvement in walking and usual activities of daily living than those who had received ≥2 prior TNFis. These data may suggest that switching to TCZ earlier in the course of disease (i.e., after an initial inadequate response to a TNFi) may be a more effective strategy than switching to another TNFi in terms of improvement in HRQOL.
The Corrona registry provides a unique opportunity to examine clinical outcomes in a large, population-based cohort of patients with established RA treated with TCZ monotherapy in routine clinical practice. The observational nature of the study and the inclusion of patients regardless of disease duration, disease severity or prior medication history (except prior use of TCZ) allowed evaluation of the efficacy of TCZ monotherapy in routine management of RA. Additionally, examination of both clinical disease activity outcomes and PROs in the same real-world cohort of patients provides a more holistic view of the efficacy of TCZ in the management of RA.
A general limitation of real-world observational studies is the concern that patients enrolled in registries may not be representative of patients observed elsewhere in general practice. However, a previous study found Medicare patients in the Corrona registry were similar to the national Medicare RA population in terms of demographic characteristics, suggesting that data from patients in Corrona may be generalizable to the population of patients with RA in the United States [18]. In this study cohort, the sample size for TNFi-naive patients was small, which may be due in part to the use of TCZ more often as a second-line biologic following an inadequate response to ≥1 TNFi [8]. Additionally, the effects of disease duration, patient adherence to treatment, and treatment persistency were not assessed in this study. Specifically, the sample sizes for patients who switched from TCZ to a new biologic, added a csDMARD, or discontinued TCZ without switching were small, which inhibited subanalyses comparing outcomes in these patient groups with patients who continued TCZ monotherapy through the 1-year study endpoint. Furthermore, follow-up time was limited to 1 year; longer follow-up is necessary to assess the long-term effectiveness of TCZ monotherapy in managing RA disease activity and HRQOL. Finally, this study was an observational study that assessed data from patients with RA treated with TCZ monotherapy without a control group; therefore, no conclusions can be drawn in comparison to other available treatment options for RA.
Conclusions
In conclusion, patients with active, refractory RA and an inadequate response to csDMARDs and/or TNFis who initiated TCZ monotherapy experienced significant improvements in disease activity and HRQOL. This study demonstrated the efficacy of TCZ monotherapy in patients with RA, regardless of prior TNFi exposure. In addition, our results demonstrate that using validated measures of disease activity in combination with PROs provides a more holistic view of the efficacy of treatment with TCZ monotherapy in patients with RA cared for in typical clinical practice.
References
Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906.
Scott DL, Steer S. The course of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:943–67.
Deshpande PR, Rajan S, Sudeepthi BL, Nazir A. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–44.
Her M, Kavanaugh A. Patient-reported outcomes in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:327–34.
Kalyoncu U, Dougados M, Daures JP, Gossec L. Reporting of patient-reported outcomes in recent trials in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2009;68:183–90.
Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72:43–50.
Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625–39.
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind, randomised controlled trial. Lancet. 2004;363:675–81.
Backhaus M, Kaufmann J, Richter C, Wassenberg S, Roske AE, Hellmann P, et al. Comparison of tocilizumab and tumor necrosis factor inhibitors in rheumatoid arthritis: a retrospective analysis of 1603 patients managed in routine clinical practice. Clin Rheumatol. 2015;34:673–81.
Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50.
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96.
Kievit W, Fransen J, Oerlemans AJ, Kuper HH, van der Laar MA, de Rooij DJ, et al. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann Rheum Dis. 2007;66:1473–8.
Wolfe F, Michaud K, Dewitt EM. Why results of clinical trials and observational studies of antitumour necrosis factor (anti-TNF) therapy differ: methodological and interpretive issues. Ann Rheum Dis. 2004;63:ii13–7.
Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(10):96–9.
Kremer JM. The CORRONA database. Clin Exp Rheumatol. 2005;23:S172–7.
Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, et al. Linkage of a de-identified united states rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken). 2014;66:1790–8.
Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64:640–7.
Curtis JR, Yang S, Chen L, Pope JE, Keystone EC, Haraoui B, et al. Determining the absolute change in the clinical disease activity index (CDAI) to define a minimally important difference. Arthritis Rheum. 2013;65:2866.
Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20:557–60.
Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34:280–9.
Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Strand V, Boers M, Idzerda L, Kirwan JR, Kvien TK, Tugwell PS, et al. It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol. 2011;38:1720–7.
Wolfe F, Pincus T. Listening to the patient: a practical guide to self-report questionnaires in clinical care. Arthritis Rheum. 1999;42:1797–808.
Khan NA, Yazici Y, Calvo-Alen J, Dadoniene J, Gossec L, Hansen TM, et al. Reevaluation of the role of duration of morning stiffness in the assessment of rheumatoid arthritis activity. J Rheumatol. 2009;36:2435–42.
Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQoL (EQ-5D). Rheumatology (Oxford). 1997;36:551–9.
Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis. 2014;73:803–9.
Kojima T, Yabe Y, Kaneko A, Takahashi N, Funahashi K, Kato D, et al. Importance of methotrexate therapy concomitant with tocilizumab treatment in achieving better clinical outcomes for rheumatoid arthritis patients with high disease activity: an observational cohort study. Rheumatology (Oxford). 2015;54:113–20.
Acknowledgements
This study is sponsored by Corrona, LLC. Corrona, LLC has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Bristol-Myers Squibb, Crescendo, Eli Lilly and Company, Genentech, GSK, Horizon Pharma USA, Janssen, Momenta Pharmaceuticals, Novartis, Pfizer, Roche, and UCB. Financial support for this analysis was provided by Genentech, Inc. Article processing charges were funded by F. Hoffmann-La Roche, Ltd. Support for third-party writing assistance for this manuscript, furnished by Elizabeth Ohneck, PhD, of Health Interactions, Inc, was provided by F. Hoffmann-La Roche, Ltd.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Drs Harrold and Reed had full access to all of the data and take complete responsibility for the integrity of the data.
Disclosures
Leslie R. Harrold is an employee of University of Massachusetts Medical School and Corrona, LLC, is a shareholder of Corrona, LLC, and has received research support from Pfizer. Ani John is an employee of Genentech, Inc. Jennie Best is an employee of Genentech, Inc. Steve Zlotnick is an employee of Genentech, Inc. George W. Reed is an employee and shareholder of Corrona, LLC. Tmirah Haselkorn is a paid consultant to Genentech, Inc. Chitra Karki is an employee of Corrona, LLC. YouFu Li is an employee of University of Massachusetts Medical School. Joel M. Kremer is an employee and shareholder of Corrona, LLC, is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Inc., GSK, Lilly, Pfizer, Regeneron, and Sanofi, and has received research support from AbbVie, Genentech, Inc., Lilly, Novartis, and Pfizer. Jeffrey D. Greenberg is an employee and shareholder of Corrona, LLC and a consultant for Eli Lilly and Company, Genentech, Inc., Janssen, Novartis, and Pfizer.
Compliance with Ethics Guidelines
Ethics approvals for this study were obtained from New England Independent Review Board (IRB# 120160610) for private practice sites and local institutional review boards of participating academic sites. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/F70CF06037CEDF60.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Harrold, L.R., John, A., Reed, G.W. et al. Impact of Tocilizumab Monotherapy on Clinical and Patient-Reported Quality-of-Life Outcomes in Patients with Rheumatoid Arthritis. Rheumatol Ther 4, 405–417 (2017). https://doi.org/10.1007/s40744-017-0081-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-017-0081-3