Abstract
Introduction
The purpose of the current study was to examine the painful elbow, and in particular enthesitis, in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using clinical examination, ultrasonography (US) and magnetic resonance imaging (MRI).
Methods
Patients with elbow pain (11 with PsA and 9 with RA) were recruited. Clinical examination, US and MRI studies were performed on the same day. For enthesitis, the common extensor and flexor insertions and the triceps insertion were imaged (20 patients, giving a total of 60 sites with comparative data). Imaging was performed with the radiologists blinded to the diagnosis and clinical findings. US was used to assess ‘inflammatory activity’ (Power Doppler signal, oedema, tendon thickening and bursal swelling) and ‘damage’ (erosions, cortical roughening and enthesophytes). MRI was used to assess ‘inflammation’ (fluid in paratenon, peri-entheseal soft-tissue oedema, entheseal enhancement with gadolinium, entheseal oedema and bone oedema) and ‘damage’ (erosion, cortical roughening and enthesophyte).
Results
Complete scan data were not available for all patients as one patient could not tolerate the MRI examination. No significant differences in imaging scores were found between PsA and RA. Analysis of damage scores revealed complete agreement between US and MRI data in 43/55 (78%) comparisons; in 10/55 (18%) cases the US data were abnormal but the MRI data normal; in 2/55 (4%) cases, the MRI data were abnormal and the US data normal. Analysis of the inflammation scores revealed complete agreement between US and MRI data in 33/55 (60%) comparisons; in 3/55 (5%) cases US data were abnormal but MRI data normal; in 19/55 (35%) cases the MRI data were abnormal and the US data normal. There was a poor relationship between assessments based on clinical examination and imaging studies. Readers could not accurately identify the disease from imaging findings.
Conclusion
Based on our results, at the elbow, US and MR have different roles in assessing enthesitis, with US apparently the better diagnostic tool for assessing damage and MR the better tool for assessing inflammation. In this study enthesitis and synovitis in the painful elbow were found equally in cases of established RA and PsA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enthesitis is an important aspect of spondyloarthropathy. It plays a pivotal part in the spinal changes which occur in ankylosing spondylitis (AS) and may have a similar role in the peripheral arthritis of psoriatic arthritis (PsA) and other spondyloarthropathies. Enthesitis is assessed separately in clinical studies of AS and PsA and was placed in the ‘outer circle’ (recommended for clinical trials) of domains determined for PsA at the Outcomes in Rheumatology Clinical Trials (OMERACT) meeting in Malta 2006 [1]. Further, enthesitis assessment is included in the domains in three composite measures of disease activity in PsA [2,3,4].
RA is a different disease to PsA in terms of genetics, pathogenesis, presentation and outcome. It has been hypothesised that the primary pathological lesion occurs in the synovium in RA and at the enthesis in PsA [5]. According to this hypothesis, synovitis in PsA is considered to be secondary to the extra-capsular disease. On the other hand, a number of authors have recognised the occurrence of enthesitis as part of the RA disease spectrum [6,7,8]. It is therefore clear that, by the time the patient presents, both synovitis and enthesitis may occur in both conditions, occasionally making it difficult to distinguish between them on radiological grounds [9]. However, in established disease, differences have been described—symmetrical involvement, periarticular osteopenia and marginal erosions in RA; relatively preserved bone stock, paramarginal erosions, osteolysis, perisostitis and periarticular new bone in PsA [10].
A clinical enthesis index specific to PsA has recently been developed [11]. This index examines tenderness at six sites: lateral epicondyles of the humerus, medial condyles of the femur and the insertion of the Achilles tendon. The proximity of the lateral epicondyle of the humerus to the elbow joint (EJ) exemplifies the difficulties of separating entheseal from articular tenderness, particularly when both occur together. The aim of the study reported here was threefold: firstly, to examine the relationship between enthesitis and synovitis by comparing data from clinical and imaging examinations of this anatomical area in PsA and RA; secondly, to examine the relationship between clinical enthesitis and enthesitis found on imaging; thirdly, to compare ultrasound (US) and magnetic resonance imaging (MRI) in terms of identifying features of enthesitis. We hypothesised (1) that synovitis would be more prominent in RA, and enthesitis in PsA; (2) that there would be a poor relationship between clinical enthesitis and enthesitis identified by US and MRI; (3) that US would provide more information than MRI on enthesitis at the elbow.
Methods
Full ethical committee approval was given for this study, and all patients provided their signed, informed consent to participate (Bradford REC approval 09/H1302/113). Subjects were seen in rheumatology out-patient clinics and, after consent procedures had been completed, examined using a standard clinical protocol. Patients with a physician diagnosis of PsA and RA were eligible if they complained of pain in the elbow region. The protocol consisted of gathering sufficient clinical information to assess the Classification of Psoriatic Arthritis (CASPAR) criteria [12], an acute phase marker and a swollen joint count. It included an assessment of the entheses at the elbow, namely, the common extensor origin (CEO) at the lateral epicondyle, the common flexor origin (CFO) at the medial epicondyle and the triceps insertion. Pressure was exerted at the enthesis sufficient to blanch the thumb nail of the examiner (approximately 4 kg). In addition, the examiner assessed the presence of soft-tissue swelling at the enthesis and performed ‘stress’ testing at each site (for medial and lateral epicondyles, resisted extension and flexion of the wrist with the arm fully extended; for triceps, resisted extension of the elbow).
Active inflammatory arthritis in the EJ was assessed by palpation. For the radio-humeral component (RHJ) the examiner faced the patient and grasped the hand of the patient in a gentle handshake (with the right hand for the right RHJ and the left hand for the left RHJ). With the thumb of the other hand the examiner then palpated over the RHJ while supinating/pronating the forearm. Tenderness and pain on movement indicated a positive test. Swelling at this location could not reliably be identified. For the EJ the examiner palpated the joint line anteriorly and the groove between the lateral epicondyle and the olecranon process, noting soft-tissue swelling and tenderness at this point. Articular damage was scored if the patient had more than 50% loss of movement and/or had bony swelling of the joint margins.
The US and MRI examinations were carried out on the same day as the clinical evaluation, with the US images assessed by one radiologist and the MRI images assessed by a second radiologist; each radiologist was ‘blinded’ to the diagnosis and to the findings of the ‘other’ imaging modality. After each radiological examination, the radiologists made a decision on the diagnosis based on their respective interpretation of the imaging data. Each radiologist had over 15 years of experience in musculoskeletal radiology. A third radiologist performed a second reading of both the MRI and (static) US scans; this radiologist was also blinded to the diagnosis and to the findings in each imaging modality.
Ultrasound Protocol
The scans were performed on a Siemens S2000 machine using a 10-15 MHz linear probe (Siemens AG, Munich, Germany). The sonographic assessments were made at the CEO, the CFO and the attachment of the triceps tendon at the olecranon. The CEO (anatomically at the lateral epicondyle of the elbow) was examined with the patient seated and the hand resting on the knee with the elbow slightly flexed and the wrist in gentle internal rotation. The CFO (anatomically at the medial epicondyle of the elbow) was examined with the arm fully extended and the arm resting on the knee with the palm facing anteriorly. Grey scale imaging in the longitudinal and transverse planes was used to assess the enthesis for the presence of erosions, enthesophytes, entheseal thickening and perientheseal soft-tissue oedema. Lesions were scored as present (score 1) or absent (score 0). An erosion was defined as a step-down cortical contour defect seen in two planes and measuring ≥2 mm in depth. An enthesophyte was defined as a step-up bony prominence at the end of a normal bone contour and forming a bony spur seen within the tendinous portion of the enthesis. Entheseal thickening was scored as present if there was a discrepancy between the thickness at the contralateral enthesis or if the normally smooth entheseal contours appeared to be bulky. Peri-entheseal soft-tissue oedema was scored as present if there was compressible fluid within the soft tissues on the outer margin of the enthesis. Assessment of entheseal vascularisation was performed using Power Doppler mode, with the pulse repetition frequency set at 600. Entheseal vascularity was scored as present (score 1) or absent (score 0).
The following scores were calculated as an approximation of ‘inflammation’ and ‘damage’ at the enthesis:
-
(1)
Inflammation: vascularisation, fluid in paratenon, perientheseal soft-tissue oedema, altered echogenicity and thickening of tendon (score range 0–5).
-
(2)
Damage: erosion, cortical roughening and enthesophyte (score range 0–3).
For the joint, both grey scale of effusion/synovitis and Power Doppler mode were used in assessment (articular score, range 0–2).
This protocol is in keeping with the recommendations of the OMERACT US in Spondyloarthropathy Study Group [13].
MRI Protocol
Scans were performed on a Siemens Avanto 1.5T scanner (Siemens AG). Patients were imaged prone in the ‘superman position’ with the arm extended into the centre of the bore using a wrap-around coil.
For the purposes of this study, the following sequences were performed:
-
(1)
Axial T1-weighted. Repetition time(TR)/echo time (TE), 300/24 ms; echo-train length (ETL), 1; matrix, 144 × 192; field of view (FOV), 11 cm; number of excitations, 1; slice thickness 3 mm with 0.3-mm interslice gap.
-
(2)
Coronal T1-weighted. TR/TE, 333–361/24 ms; ETL, 1; matrix, 218 × 257; FOV, 13 cm; number of excitations, 1; slice thickness 3 mm with 0.3-mm interslice gap.
-
(3)
Axial T2 fat-saturated. TR/TE, 2290–4800/80– ms; ETL, 9–16; matrix, 192 × 256; FOV, 12 cm, number of excitations, 1; slice thickness, 3 mm with 0.3-mm interslice gap.
-
(4)
Coronal short-tau T1 inversion recovery (STIR). TR/TE, 4630–5130/34 ms; ETL, 7; matrix, 205 × 256; FOV, 13 cm, number of excitations, 1; slice thickness, 3 mm with 0.3-mm interslice gap.
A standard dose of 0.2 mL/kg body weight of gadoterate dimeglumine (Dotarem; Guerbet, Aulney-sous-Bois, France) was administered intravenously by hand injection, followed by a flush of 20 mL saline solution (0.9%). T1 fat-saturated sequences in three orthogonal planes after gadolinium administration (TR/TE, 580–710/24 ms; ETL, 1; matrix, 218 × 256; FOV, 10 cm; number of excitations, 1; slice thickness, 3 mm with 0.3-mm interslice gap) were subsequently obtained.
This protocol is in keeping with the recommendations of the OMERACT MRI Study Group for PsA [14].
Images were scored in a similar fashion to the US images as described above. Scores were calculated as an approximation of ‘inflammation’ and ‘damage’ at the enthesis, as follows:
-
(1)
Inflammation: fluid in paratenon, peri-entheseal soft-tissue oedema, entheseal enhancement with gadolinium, entheseal oedema, and bone oedema (score range 0–5).
-
(2)
Damage: erosion, cortical roughening and enthesophyte (score range 0–3).
Further, images were scored for synovitis in the elbow and radio-humeral joints, as defined by the presence of a joint effusion and synovial enhancement following gadolinium administration (Articular score, range 0–2).
Statistics
All statistics were carried out using SPSS software v 21.0 (IBM Corp., Armonk, NY). Continuous data were compared using non-parametric statistics, dichotomous data using Chi-squared tests and where insufficient cell numbers were found, we performed a Fisher’s exact test. Agreement between readers was examined with the kappa (κ) statistic. By general consensus, kappa values of <0 indicate no agreement, 0–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement and 0.81–1 almost perfect agreement. Intra-class correlation was used to compare the scores of the two observers.
Results
The study included 20 patients. Of these, 11 had with PsA [5 males, 6 females; mean age 48.9 years; mean duration of disease 6.8 years; mean C-reactive protein (CRP) 2.3 mg/dL; mean swollen joint count 3.4], and nine had RA (4 males, 5 females; mean age 61.9 years; mean duration of disease 12.4 years; mean CRP 10.5 mg/dL; mean swollen joint count 3.5). All RA patients fulfilled the 1987 criteria for RA [15] and eight of the 11 patients with PsA fulfilled the CASPAR criteria.
Clinical Examination
All patients had a painful elbow. Generally more patients in the PsA group had clinical enthesitis (PsA: N = 10 and 8 at the CEO and CFO, respectively; RA: N = 6 and 4 for CEO and CFO, respectively), and more patients with RA had articular involvement (PsA: N = 4 and 3 for EJ and RHJ, respectively; RA: N = 8 and 3 for EJ and RHJ, respectively). The only statistically significant difference between the diagnostic groups was for EJ tenderness and swelling (Chi-squared test 5.7; p = 0.28). Very few cases of clinical articular damage were found [2 cases of PsA and 3 cases of rheumatoid arthritis (RA)].
Imaging Scores by Disease Group
Complete imaging data were not available for all patients due to one patient not tolerating the MRI procedure; overall, data on 55/60 comparisons at the three entheseal sites were available. Scores for both enthesitis and articular involvement by diagnosis are given in Table 1. For both diseases the highest scores were found for inflammation and damage at the CEO by both imaging modalities (US: PsA mean inflammation and damage scores of 1.82 and 0.55, respectively; RA mean inflammation and damage scores of 1.57 and 1.50, respectively; MRI: PsA mean inflammation and damage scores of 2.55 and 0.55, respectively; RA mean inflammation and damage scores of 1.88 and 0.50 respectively). None of the differences were statistically significant.
Relationship Between US and MRI findings at the Enthesis
The scores for damage differed from those for inflammation. For damage scores, complete agreement between US and MRI data was found in 43/55 (78%) comparisons; in 10/55 (18%) cases the US data were abnormal but the MRI data were normal; in 2/55 (4%) cases the MRI data were abnormal and the US data were normal.
For inflammation scores complete agreement between US and MRI data was found in 33/55 (60%) comparisons; in 3/55 (5%) cases the US data were abnormal but the MRI were normal; in 19/55 (35%) cases the MRI data were abnormal and US data were normal. Thus, US appeared to be the more sensitive imaging modality for evaluating damage and MRI the more sensitive imaging modality for evaluating inflammation. Overall unadjusted kappa scores between US and MRI data were 0.34 for damage scores and 0.23 for inflammation scores.
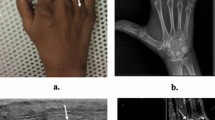
Examples of US and MR images at the elbow are shown in Figs. 1–6.
a Coronal magnetic resonance (MR) short-tau T1 inversion recovery (STIR) imaging. Normal common extensor origin (CEO) with homogenous low signal throughout (arrowhead). Note the signal at the CEO cannot be identified separately from the lateral collateral ligament (arrow). There is a small effusion in the joint. b Ultrasound image. Longitudinal section through the normal CEO. Fibrils are apparent within the ligament substance. There is a bright, regular, echogenic paratenon (arrow), and the slice also shows the edge of the radiocarpal joint with normal fat pad (star)
Coronal MR T1 fat-saturated sequence plus contrast. Severe lateral epicondylitis with enhancing, inflammatory tissue between the oedematous fibres of the common extensor origin (arrowheads) and multiple small erosions in the lateral epicondyle (arrow) are visualized. The lateral collateral ligament is shown separately from the CEO and is oedematous with a small erosion at the origin
a Ultrasound. Longitudinal section through the common extensor origin. Normal cortical margins and the fibrils of the CEO are well shown, but there is a partial thickness tear of the ligament with intra-substance extension (arrowheads). b Coronal MR T1 fat-saturated sequence plus contrast. There is a partial thickness tear of the common extensor origin with distal retraction of the torn fibres (arrowheads) and normal lateral collateral ligament (arrows)
Relationship Between the Results of Clinical Examinations and Imaging Findings
Overall there was a poor agreement between the clinical findings and the imaging results. Further, no significant relationship was found between clinical enthesitis and the presence of inflammation in the components of the EJ (data not shown). Kappa scores for agreement between the clinical and imaging results were calculated, aggregated for all sites. For the US inflammation score (any positive) and clinical tests, the kappa scores for agreement were 0.1, 0.16 and 0.12 for stressing, swelling and tenderness, respectively. For the MRI inflammation scores and clinical tests, the kappa scores for agreement were 0.28, 0.04 and 0.11 for stressing, swelling and tenderness, respectively. Levels of agreement at individual locations were also generally poor, except for the CEO (stress) and MR inflammation (κ = 0.41), and for CEO tenderness and MR inflammation (κ = 0.28).
Agreement Between Observers on the Imaging
Agreement between observers was slight to moderate when aggregated across all entheseal sites, and for each modality (see Table 2). Kappa scores were highest for vascularisation (Power Doppler signal) detected by US (κ = 0.51) and entheseal enhancement detected by MRI (κ = 0.32). Interclass correlation (ICC) scores were also modest for enthesitis, with the largest scores found for the inflammation scores in the MRI studies (Triceps 0.59; CEO 0.42; CFO 0.39). However, ICC scores were good for agreement on MR inflammation in the EJ (κ = 0.86) and US inflammation in the EJ (κ = 0.52).
Observers’ Diagnosis Based on Imaging
There was a poor relationship between the diagnosis of the primary radiologist, based on imaging findings, and the clinical diagnosis. The radiologists were unable to distinguish between RA and PsA on the basis of imaging findings. The radiologist interpreting the US scans ‘misclassified’ 10/17 cases, and the radiologist interpreting the MRI scans ‘misclassified’ 7/16 cases. The kappa scores were 0.29 and 0.08 for the level of agreement between US and clinical diagnoses and between MRI and clinical diagnoses, respectively.
Discussion
The aim of this study was to focus on the entheses around the EJ in terms of clinical and imaging findings. US and MR imaging, while giving contrasting information on inflammation and damage, remain complementary modalities. The relationship between clinical and imaging enthesitis and synovitis was poor. Further, it was impossible to accurately identify a disease group from the imaging results alone, either with US or MRI.
To place some of these findings in context it is worth noting that abnormalities at the entheses around the elbow have been demonstrated in asymptomatic individuals [16] and in patients with RA in whom clinical inflammation has subsided [7]. Both these factors are likely to confound studies looking at the relationship between clinical and imaging findings at the enthesis, and in comparing imaging and clinical findings between diseases. Eshed et al. recognised that while MRI is sensitive for enthesopathy, the findings lack specificity and differentiating between the different aetiologies of enthesitis on the basis of their MRI characteristics is not always possible [17]. Our study would appear to confirm this conclusion and may suggest that the process of entheseal inflammation is similar in both RA and PsA. US appeared to be more sensitive for detecting damage and MRI more sensitive for detecting inflammation, although a ‘gold standard’, such as histology, was not available. Nevertheless, this result is to be expected as the resolution of MRI is much lower than that of US and, consequently, the former is relatively insensitive to areas of bony abnormality, such as enthesophytes or small erosions.
The discrepancy between clinical and imaging enthesitis in spondyloarthropathy is not a novel finding [18,19,20]. This may in part be due to the fact that clinical examination and imaging studies are measuring different things. For example, US cannot visualise osteitis, yet osteitis may cause tenderness at the enthesis. Osteitis, or bone oedema, can be visualised at the enthesis in spondyloarthropathy using MRI, and this has been interpreted as indicating enthesitis [21]. However, it was clear that the relationship between the MRI and clinical findings in the current study were poor. In a similar study by Aydin and colleagues in the knee found an equally poor relationship between imaging and clinical scores [20]. In the current study one reason for the poor association could have been the low prevalence of abnormality, particularly in the triceps insertion and CFO, and this may disadvantage the kappa statistic. That a ‘good’ relationship between MR inflammation and CEO stressing was found reflects the value of this test and its relationship to osteitis at the entheseal insertion.
It had been thought that this study would show a clear difference between the clinical and imaging findings of RA and PsA. However, it must be noted that all the patients included in this study had established disease, which may have ‘blurred’ the differences between them. Further, the mean age of the patients meant that degenerative enthesopathy could be a confounding factor. It is possible that the greater age of the RA patients enrolled in our study could have influenced the frequency of degenerative changes at the enthesis but, notably, inflammatory changes were also found equally in these diseases. The commonest abnormalities at each of the three entheseal sites were found at the CEO, and ‘lateral epicondylalgia’ is a common clinical presentation in primary care in the UK [22].
How do these results inform the use of the Leeds Enthesitis Index, where the lateral epicondyle of the elbow is one of the three sites examined? The poor relationship between clinical examination and imaging findings has been confirmed but, on the positive side, there was no relationship between inflammation in the joint and inflammation in the adjacent enthesis on imaging, and no relationship between pain at the enthesis and underlying articular inflammation. The anatomical relationship of the radio-humeral joint, its capsular insertion and the CEO suggest that inflammation in the EJ cannot extend to the CEO without disruption of the articular capsule at this point, and which in itself may represent a process of entheseal inflammation at the capsular insertion. Discriminating between synovitis and enthesitis at the elbow, and at other joints, may therefore, and particularly in established disease, be a futile exercise because of the inter-relationship between the two processes.
Conclusions
In summary, a poor relationship was found between clinical and imaging assessments, and between imaging modalities. US seemed the better modality for assessing damage at the enthesis, and MRI the better modality for assessing inflammation. However, MRI is generally the more sensitive of these two modalities and should be considered as the next line of investigation following a normal US result. No differences were found between PsA and RA in terms of enthesitis.
References
Gladman DD, Mease PJ, Strand V, Healy P, Helliwell PS, Fitzgerald O, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34(5):1167–70.
Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis. 2013;72(6):986–91.
Mumtaz A, Gallagher P, Kirby B, Waxman R, Coates LC, Veale JD, et al. Development of a preliminary composite disease activity index in psoriatic arthritis. 2011;70(2):272–7.
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. 2010;69(1):48–53.
McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352(9134):1137–40 (see comment).
Helliwell PS, Porter G, group Cs. Sensitivity and specificity of plain radiographic features of peripheral enthesopathy at major sites in psoriatic arthritis. Skelet Radiol. 2007;36(11):1061–6.
Ibrahim GH, Groves C, Chandramohan M, Valle R, Beltran A, Reyes B, et al. Clinical and ultrasound validation of the Leeds Enthesitis Index in psoriatic arthritis and rheumatoid arthritis. ISRN Rheumatol. 2011;731917:5. doi:10.5402/2011/731917.
Frediani B, Falsetti P, Storri L, Allegri A, Bisogno S, Baldi F, et al. Ultrasound and clinical evaluation of quadricipital tendon enthesitis in patients with psoriatic arthritis and rheumatoid arthritis. Clin Rheumatol. 2002;21(4):294–8.
Marzo-Ortega H, Tanner SF, Rhodes LA, Tan AL, Conaghan PG, Hensor EMA, et al. Magnetic resonance imaging in the assessment of metacarpophalangeal joint disease in early psoriatic and rheumatoid arthritis. Scan J Rheumatol. 2009;38(2):79–83.
McGonagle D. Imaging the joint and enthesis: insights into pathogenesis of psoriatic arthritis. Ann Rheum Dis. 2005;64[Suppl II]:ii58–60. doi:10.1136/ard.2004.034264.
Healy P, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis:assessment of existing measures and development of an instrument specific for psoriatic arthritis. Arthritis Care Res. 2008;59(5):686–91.
Taylor WJ, Gladman DD, Helliwell PS, Marchesoni A, Mease PJ, Mielants H. Classification criteria for psoriatic arthritis. Arthritis Rheum. 2006;54(8):2665–73.
Terslev L, Naredo E, Iagnocco A, Balint PV, Wakefield RJ, Aegerter P, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res. 2014;66(5):741–8.
McQueen F, Lassere M, Bird P, Haavardsholm EA, Peterfy C, Conaghan PG, et al. Developing a magnetic resonance imaging scoring system for peripheral psoriatic arthritis. J Rheumatol. 2007;34(4):859–61.
Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Levin D, Nazarian L, Miller T, O’Kane P, Feld R, Parker L, et al. Lateral epicondylitis of the elbow: US findings. Radiology. 2005;237(1):230–4.
Eshed I, Bollow M, McGonagle DG, Tan AL, Althoff CE, Asbach P, et al. MRI of enthesitis of the appendicular skeleton in spondyloarthritis. Ann Rheum Dis. 2007;66(12):1553–9.
D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. 2003;48(2):523–33.
Balint P, Kane D, Wilson H, McInnes I, Sturrock R. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61(10):905–10.
Aydin S, Tan AL, Hodgson R, Grainger A, Emery P, Wakefield RJ, et al. Comparison of ultrasonography and magnetic resonance imaging for the assessment of clinically defined knee enthesitis in spondyloarthritis. Clin Exp Rheumatol. 2013;31:933–6.
McGonagle D, Gibbon W, O’Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondylarthropathy. Arthritis Rheum. 1998;41(4):694–700.
Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964–8.
Acknowledgements
The study was part funded by the Bradford Radiology Discretionary Fund. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published. Dr. Helliwell conceived the study, collected data and undertook the analysis; Dr. Aslam collected clinical study data. All authors have reviewed the paper.
Author contributions
Dr. Helliwell is the guarantor of integrity of the entire study, established the study concepts and designed the study; literature research is not applicable to this study; Dr. Helliwell and Dr. Aslam performed the clinical studies; Dr. Groves performed the ultrasound examinations; Dr. Chandramohan interpreted the MRI images and Dr. Chew read both the ultrasound and MRI images; Dr. Helliwell performed the experimental studies/data analysis and the statistical analysis. All authors contributed to manuscript preparation and editing.
Disclosures
Clare Groves, Muthusamy Chandramohan, Ne Siang Chew, Tariq Aslam and Philip S Helliwell declare that they have nothing to disclose.
Compliance with Ethics Guidelines
Full ethical committee approval was given for this study, and all patients provided signed, informed consent to take part (Bradford REC approval 09/H1302/113).
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/CD77F06057687EB8.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Groves, C., Chandramohan, M., Chew, N.S. et al. Clinical Examination, Ultrasound and MRI Imaging of The Painful Elbow in Psoriatic Arthritis and Rheumatoid Arthritis: Which is Better, Ultrasound or MR, for Imaging Enthesitis?. Rheumatol Ther 4, 71–84 (2017). https://doi.org/10.1007/s40744-017-0053-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-017-0053-7