Abstract
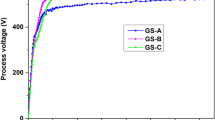
This study investigated the successful performance of acidic electroless Ni–P coatings on brass alloy from the nickel sulphamate bath. Three complexing agents (i.e., sodium acetate, acetic acid, and sodium citrate) were evaluated in a practical optimized sulphamate bath. Then, the corrosion behavior of coated samples was studied by potentiodynamic polarization (DC) and electrochemical impedance spectroscopy (EIS) methods in a 3.5 wt% NaCl solution. Moreover, the structure and chemical composition of the coated samples were characterized by X-ray diffraction (XRD) and energy dispersive spectroscopy (EDS), respectively. In addition, the morphology of the coatings was examined through scanning electron microscopy (SEM), as well as investigating properties such as thickness, grain size, and actual capacitance using computational methods. The results revealed that the structure and corrosion behavior of coatings were strongly influenced by both practical conditions and complexing agent factors. Low and medium phosphorus coatings with nanocrystalline and semi-crystalline structures were developed according to EDS and XRD results. Additionally, SEM images and electrochemical results demonstrated low phosphorous Ni–P coatings from baths containing sodium acetate with a cauliflower structure, as well as an acetic acid complexing agent with a dense structure and highly acceptable granulation which had the best anti-corrosion behavior (RP = 46,369 Ω cm2 & icorr= 0.56 \(\mathrm{\mu A}/{\mathrm{cm}}^{2}\) and RP = 44,561 Ω cm2 & icorr= 0.7 \(\mathrm{\mu A}/{\mathrm{cm}}^{2}\), respectively) compared to a bare sample (Rp = 3687 Ω cm2 & icorr= 2.72 \(\mathrm{\mu A}/{\mathrm{cm}}^{2}\)).







Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Brock J, Zaroog OS (2017) Copper alloys: corrosion, reference module in materials science and materials engineering. Elsevier Inc, Cambridge
Hsu RC, Huang CH, Yu MS (2018) Mechanical and fatigue properties of electro-less Ni-P coating on brass substrates by plasma-etched pretreatment. Int J Fatigue. https://doi.org/10.1016/j.ijfatigue.2018.02.030
Zhoua P, Hutchisonb MJ, Erningc JW, Scullyb JR, Oglea K (2017) An in situ kinetic study of brass dezincification and corrosion. Electrochim Acta. https://doi.org/10.1016/j.electacta.2017.01.078
Tuck CDS, Powell CA, Nuttall J (2010) Corrosion of copper and its alloys. Shreir’s Corros. https://doi.org/10.1016/B978-044452787-5.00094-9
Imani M, Dastanpoor E, Enayati MH, Basak AK (2021) Thermodynamic prediction of phase formation in Ni–P alloy system during mechanical alloying: comparison with electroless plating technique. Met Mater Int. https://doi.org/10.1007/s12540-019-00586-8
Novakovic J, Vassiliou P, Samara KL, Argyropoulos TH (2006) Electroless NiP–TiO2 composite coatings: their production and properties. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2006.01.005
Huang YS, Zeng XT, Hu XE, Liu FM (2004) Corrosion resistance properties of electroless nickel composite coatings. Electrochim Acta. https://doi.org/10.1016/j.electacta.2004.04.023
Nwosu N, Davidson A, Hindle C, Barker M (2012) On the influence of surfactant incorporation during electroless nickel plating. Ind Eng Chem Res. https://doi.org/10.1021/ie202625n
Zhang B (2016) Amorphous and nano alloys electroless depositions technology, composition, structure and theory. chemical industry press. Elsevier Inc, Cambridge, pp 3–48
Sudagar J, Lian J, Sha W (2013) Electroless nickel, alloy, composite and nano coatings—a critical review. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2013.03.107
Zhao H, Liu L, Zhu J, Tang Y, Hu W (2007) Microstructure and corrosion behavior of electrodeposited nickel prepared from a sulphamate bath. Mater Lett. https://doi.org/10.1016/j.matlet.2006.07.178
Sivasakthi P, Sekar R, Ramesh Bapu GNK (2015) Pulse electrodeposited nickel using sulphamate electrolyte for hardness and corrosion resistance. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2015.06.019
Islam M, Azhar MR, Fredj N, Burleigh TD, Oloyede OR, Almajid AA, Shah SI (2015) Influence of SiO2 nanoparticles on hardness and corrosion resistance of electroless Ni–P coatings. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2014.11.044
Islam M, Azhar MR, Fredj N, Burleigh TD (2013) Electrochemical impedance spectroscopy and indentation studies of pure and composite electroless Ni–P coatings. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2013.09.057
Ahmadi Ashtiani A, Faraji S, Amjad Iranagh S, Faraji AH (2017) The study of electroless Ni–P alloys with different complexing agents on Ck45 steel substrate. Arab J Chem. https://doi.org/10.1016/j.arabjc.2013.05.015
Chintada VB, Koona R, Raju Bahubalendruni MVA (2021) State of art review on nickel-based electroless coatings and materials. J Bio- Tribo-Corros. https://doi.org/10.1007/s40735-021-00568-7
Wu CY, Chen YH, Tang YK, Lin EJ, Lin YX, Wang JY, Zhuang WX, Lee CH, Chiu CY, Yeh CY, Hslao CY, Cheng ML, Liu AL, Liu CY (2020) Effect of chemical additives in the plating bath on surface corrosion resistance of Ni(P). J Electron Mater. https://doi.org/10.1007/s11664-019-07626-4
Mallory GO (1999) Electroless plating: fundamentals and applications. American Electroplaters and Surface Finishers Society, Orlando, pp 1–56
Riedel W (1991) Electroless nickel plating, 2nd edn. ASM International, Almere, pp 204–220
Zhang B (2016) Amorphous and nano alloys electroless depositions technology, composition, structure and theory. Elsevier Inc, Cambridge, pp 323–381
Mallory GO (1999) Electroless plating: fundamentals and applications. American Electroplaters and Surface Finishers Society, Orlando, pp 57–99
Fields W, Zickearff JR (1984) Electroless, Publication of ASM committee on EN platting
Karmakar R, Maji P, Ghosh SK (2021) A review on the nickel based metal matrix composite coating. Met Mater Int. https://doi.org/10.1007/s12540-020-00872-w
Wu WP, Jiang JJ (2017) Effect of plating temperature on electroless amorphous Ni–P film on Si wafers in an alkaline bath solution. Appl Nanosci. https://doi.org/10.1007/s13204-017-0575-x
Ansari MI, Thakur DG (2016) Effect of bath agitation on surface properties and corrosion behavior of ENi-P coatings along with annealing temperature. Eng Sci Technol Int J. https://doi.org/10.1016/j.jestch.2016.09.020
Dr-Ing NV, Mandich CEF (2003) Surface preparation of metals prior to plating. Met Finish. https://doi.org/10.1016/S0026-0576(03)90245-5
Subramanian C, Cavallaro G, Winkelman G (2000) Wear maps for titanium nitride coatings deposited on copper and brass with electroless nickel interlayers. Wear. https://doi.org/10.1016/S0043-1648(00)00380-X
Rohan JF, O’Riordan G, Boardman J (2002) Selective electroless nickel deposition on copper as a final barrier/bonding layer material for microelectronics applications. Appl Surf Sci. https://doi.org/10.1016/S0169-4332(01)00982-5
B733 – 15 (2015) Standard specification for autocatalytic (electroless) nickel-phosphorus coatings on metal. ASTM Int United States. https://doi.org/10.1520/B0733-15
Zarebidaki A, Allahkaram SR (2012) Porosity measurement of electroless Ni–P coatings reinforced by CNT or SiC particles. Surf Eng. https://doi.org/10.1179/1743294411Y.0000000087
Czagánya M, Baumlia P, Kaptay G (2017) The influence of the phosphorous content and heat treatment on the nano-micro-structure, thickness and micro-hardness of electroless Ni–P coatings on steel. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2017.06.168
Duncan RN (1996) The metallurgical structure of electroless nickel deposits: effect on coating properties. Plat Surf Finish 83:65
Salicio-Paz A, Dalmau H, Grande A, Iriarte J, Sort E, Pellicer J, Fornell E-L (2020) Impact of the multilayer approach on the tribocorrosion behaviour of nanocrystalline electroless nickel coatings obtained by different plating modes. Wear. https://doi.org/10.1016/j.wear.2020.203384
Hanachi M, Seyedraoufi ZS, Samiee M, Shajari Y (2021) Effect of phosphorus content and heat treatment temperature on microstructure and corrosion resistance of Ni–(X)P–GO nanocomposite coating on AZ31D alloy. J Bio- Tribo-Corros. https://doi.org/10.1007/s40735-020-00468-2
Niksefat V, Ghorbani M (2015) Mechanical and electrochemical properties of ultrasonic- assisted electroless deposition of Ni–B–TiO2 composite coatings. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2015.01.250
NACE Publication 6A287-HD1997, Electroless Nickel Coatings (1997 Edition)
Özkan S, Hapçı G, Orhan G, Kazmanlı K (2013) Electrodeposited Ni/SiC nanocomposite coatings and evaluation of wear and corrosion properties. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2013.06.089
Ralston KD, Birbilis N, Davies CHJ (2010) Revealing the relationship between grain size and corrosion rate of metals. Scr Mater. https://doi.org/10.1016/j.scriptamat.2010.08.035
Narayanana Tsn S, Baskaran I, Krishnaveni K, Shanmugam P (2006) Deposition of electroless Ni–P graded coatings and evaluation of their corrosion resistance. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2004.10.014
Sadreddini S, Salehi Z, Rassaie H (2015) Characterization of Ni–P–SiO2 nano-composite coating on magnesium. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2014.10.144
Lu G, Zangari G (2002) Corrosion resistance of ternary Ni-P basedalloys in sulfuric acid solutions. Electrochim Acta. https://doi.org/10.1016/S0013-4686(02)00198-6
Wronkowska AA (1993) In situ and Ex Situ characterization of passive layers on Ni1-x Px in alkaline solution. J Electrochem Soc. https://doi.org/10.1149/1.2056242
Dadvand N, Caley WF, Kipouros GJ (2004) Investigation of the corrosion behavior of electroless nickel-phosphorus coatings on AA6061 in basic solutions. Can Metall Q. https://doi.org/10.1179/cmq.2004.43.2.219
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavi, N., Sarabi Dariani, A.A. The Effect of Complexing Agent on Electroless Nickel Sulphamate Coating on Brass Alloy: Structure Characteristics & Corrosion Behavior. J Bio Tribo Corros 8, 96 (2022). https://doi.org/10.1007/s40735-022-00695-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-022-00695-9