Abstract
Heteroatoms (such as phosphorous, sulphur, oxygen and nitrogen) containing organic molecules exhibit remarkable efficiency towards corrosion inhibition. Their efficiency is attributed to the presence of lone pair of electrons and pi electrons in the molecule, due to which they are easily deposited on the metal surface. However, the organic compounds containing less electronegative heteroatoms generally show higher inhibition efficiency (IE) due to the facile migration of lone pair of electrons. Acidic solutions are widely used as electrolyte medium. The value of ∆G (Gibbs adsorption energy) reveals the nature of the inhibitors adsorb to the metal surface. Gravimetric and electrochemical analyses are extensively applied for the determination of corrosion inhibition. Similarly, surface analysis and theoretical investigation are also applied for supportive evidences. The purpose of the present review is to highlight the heteroatom-based potential corrosion inhibitors.


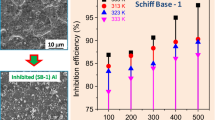
(Copyright @ Elsevier 2017)

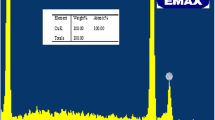
(Copyright @ Elsevier 2017)
Similar content being viewed by others
Change history
03 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40735-021-00509-4
Abbreviations
- WL:
-
Weight loss
- EIS:
-
Electrochemical impedance spectroscopy
- PDP:
-
Potentiodynamic polarization
- OCP:
-
Open circuit potential
- SEM:
-
Scanning electron microscopy
- EDS:
-
Electron dispersion x-ray spectroscopy
- QCC:
-
Quantum chemical calculation
- DFT:
-
Density function theory,
- XPES:
-
X-ray photoelectron spectroscopy
- MDS:
-
Molecular dynamic simulation
- XRD:
-
X-ray diffraction
- FTIR:
-
Fourier transform infrared
- AFM:
-
Atomic force microscopy
- EDXA:
-
Energy dispersive x-ray analysis
- GCMS:
-
Gas chromatography mass spectrophotometer
- HE:
-
Hydrogen evolution
- CTAB:
-
Cetyl Trimethyl Ammonium Bromide
References
Yadav M, Sinha RR, Kumar S (2015) Corrosion inhibition effect of spiropyrimidinethiones on mild steel in 15% HCl solution: insight from electrochemical and quantum studies. RSC Adv 5:70832–70848
Verma C, Quraishi MA, Singh A (2015) 2-Aminobenzene-1,3-dicarbonitriles as green corrosion inhibitor for mild steel in 1M HCl: electrochemical, thermodynamic, surface and quantum chemical investigation. J Taiwan Inst Chem Eng 49:229–239
Roy P, Maji T, Dey S (2015) Adsorption behaviour of gluten hydrolysate on mild steel in 1M HCl and its role as a green corrosion inhibitor. RSC Adv 5:61170–61178
Goyal M, Kumar S, Bahadur I, Verma C, Ebenso EE (2018) Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. J Mol Liq 256:565–573
Verma C, Olasunkanmi L, Ebenso EE, Quraishi M (2018) Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: a review. J Mol Liq 251:100–118
Verma DK, Khan F (2016a) Green approach to corrosion inhibition of mild steel in hydrochloric acid medium using extract of spirogyra algae. Green Chem Lett Rev 9(1):52–60
Verma DK, Khan F (2016b) Corrosion inhibition of mild steel in hydrochloric acid using extract of glycine max leaves. Res Chem Intermed 42:3489–3506
Verma DK, Khan F (2015) Corrosion inhibition of high carbon steel in phosphoric acid solution by extract of black tea. Adv. Res 5(4):1–9
Verma DK, Khan F (2016c) Corrosion inhibition of mild steel by using sulpha drugs in phosphoric acid medium: a combined experimental and theoretical approach. Am Chem Sci J 14(3):1–8
Verma DK, Khan F, Verma CB, Susai R, Quraishi MA (2017) Experimental and theoretical studies on mild steel corrosion inhibition by the grieseofulvin in 1M HCl. Eur Chem Bull 6(1):21–30
Verma DK, Ebenso EE, Quraishi MA, Verma C (2019) Gravimetric, electrochemical surface and density functional theory study of acetohydroxamic and benzohydroxamic acids as corrosion inhibitors for copper in 1M HCl. Results Phys 13:102194
Zhang J, Qiao G, Hu S, Yan Y, Ren Z, Yu L (2011) Theoretical evaluation of corrosion inhibition performance of imidazoline compoundswith different hydrophilic groups. Corros Sci 53:147–152
Musa AY, Jalgham RT, Mohamad AB (2012) Molecular dynamic and quantumchemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros Sci 56:176–183
Deng S, Li X, Xie X (2014) Hydroxymethyl urea and 1, 3-bis (hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution. Corros Sci 80:276–289
Obot I, Gasem Z (2014) Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros Sci 83:359–366
Verma C, Quraishi MA (2016) Thermodynamic, electrochemical and surface studies of dendrimers as effective corrosion inhibitors for mild steel in 1 M HCl. Anal Bioanal Electrochem 8(1):104–123
Bourichi S, Rodi YK, El Azzouzi M, Kharbach Y, Chahdi FO, Aouniti A (2017) Inhibitive effect of new synthetized imidazopyridine derivatives for the mild steel corrosion in Hydrochloric acid medium. J Mater Environ Sci 8(5):1696–1707
Ferreira ES, Giacomelli C, Giacomelli FC, Spinelli A (2004) Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83(1):129–134
Sekine I, Nakahata Y, Tanabe H (1988) The corrosion inhibition of mild steel by ascorbic and folic acids. Corros Sci 28(10):987–1001
Verma C, Olasunkanmi LO, Obot I, Ebenso EE, Quraishi M (2016a) 2, 4-Diamino-5- (phenylthio)-5 H-chromeno [2, 3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv 6:53933–53948
Verma C, Olasunkanmi L, Obot I, Ebenso EE, Quraishi M (2016b) 5-Arylpyrimido-[4, 5-b]quinoline-diones as new and sustainable corrosion inhibitors for mild steel in 1 M HCl: a combined experimental and theoretical approach. RSC Adv 6:15639–15654
Zhang Z, Tian N, Huang X, Shang W, Wu L (2016) Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: experimental and theoretical studies. RSC Adv 6:22250–22268
Khaled K (2010a) Corrosion control of copper in nitric acid solutions using some amino acids–a combined experimental and theoretical study. Corros Sci 52:3225–3234
Qin TT, Li J, Luo HQ, Li M, Li NB (2011a) Corrosion inhibition of copper by 2, 5- dimercapto-1, 3, 4-thiadiazole monolayer in acidic solution. Corros Sci 53:1072–1078
Zeng J, Zhang J, Gong X (2011) Molecular dynamics simulation of interaction between benzotriazoles and cuprous oxide crystal. Comput Theor Chem 963:110–114
Qiang Y, Zhang S, Xu S, Li W (2016) Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J Colloid Interface Sci 472:52–59
Raja PB, Qureshi AK, Rahim AA, Osman H, Awang K (2013) Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros Sci 69:292–301
Soltani N, Behpour M, Oguzie EE, Mahluji M, Ghasemzadeh MA (2015) Pyrimidine-2-thione derivatives as corrosion inhibitors for mild steel in acidic environments. RSC Adv 5:11145
Verma CB, Quraishi MA, Singh A (2016) A thermodynamical, electrochemical, theoretical and surface investigation of diheteroaryl thioethers as effective corrosion inhibitors for mild steel in 1 M HCl. J Taiwan Inst Chem Eng 58:127–140
Yadav M, Gope L, Kumari N, Yadav P (2016) Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq 216:78–86
El Azzouzi M, Aouniti A, Tighadouin S, Elmsellem H, Radi S, Hammouti B, El Assyry A, Bentiss F, Zarrouk A (2016) Some hydrazine derivatives as corrosion inhibitors for mild steel in 1.0 M HCl: weight loss, electrochemichal, SEM and theoretical studies. J Mol Liq 221:633–641
Malekmohammadi Nouri P, Attar MM (2015) Experimental and quantum chemical studies on corrosion inhibition performance of fluconazole in hydrochloric acid solution. Bull Mater Sci 38(2):499–509
Shukla SK, Quraishi MA (2009) Ceftriaxone: a novel corrosion inhibitor for mild steel in hydrochloric acid. J Appl Electrochem 39:1517–1523. https://doi.org/10.1007/s10800-009-9834-1
Singh AK, Khan S, Singh A, Quraishi SM, Quraishi MA, Ebenso EE (2013) Inhibitive effect of chloroquine towards corrosion of mild steel in hydrochloric acid solution. Res Chem Intermed 39:1191–1208. https://doi.org/10.1007/s11164-012-0677-8
Gobara M, Baraka A, Zaghloul B (2015) Inhibition of mild steel corrosion in sulfuric acid solution using collagen. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1809-0
Verma CB, Quraishi MA, Ebenso EE (2013) Green ultrasound assisted synthesis of N2, N4, N6-tris ((Pyridin-2-ylamino) methyl)-1, 3, 5-triazine-2,4,6-triamine as effective corrosion inhibitor for mild steel in 1 M hydrochloric acid medium. Int J Electrochem Sci 8:10864–10877
Jia-jun F, Su-ning L, Ying W, Xiao-dong L, Lu-de L (2011) Computational and electrochemical studies on the inhibition of corrosion of mild steel by L-Cysteine and its derivatives. J Mater Sci 46:3550–3559. https://doi.org/10.1007/s10853-011-5267-4
Muthukrishnan P, Jeyaprabha B, Tharmaraj P, Prakash P (2015) Inhibition of the corrosion of mild steel in acidic media by use of a new antipyridine derivative. Res Chem Intermed. https://doi.org/10.1007/s11164-014-1714-6
Ousslim A, Bekkouch K, Hammouti B, Elidrissi A, Aouniti A (2009) Piperazine derivatives as inhibitors of the corrosion of mild steel in 3.9 M HCl. J Appl Electrochem 39:1075–1079. https://doi.org/10.1007/s10800-008-9759-0
Awad MI (2006) Eco friendly corrosion inhibitors: inhibitive action of quinine for corrosion of low carbon steel in 1 M HCl. J Appl Electrochem 36:1163–1168. https://doi.org/10.1007/s10800-006-9204-1
Rajeswari V, Kesavan D, Gopiraman M, Viswanathamurthi P (2013) Inhibition of cast iron corrosion in acid, base, and neutral media using Schiff Base derivatives. J Surfactants Deterg 16(4):571–580
Seung-Hyun Y, Young-Wun K, Keunwoo C, Nam-Kyun K, Joon-Seop K (2013) Corrosion inhibition properties of triazine derivatives containing carboxylic acid and amine groups in 1.0 M HCl solution. Ind Eng Chem Res. https://doi.org/10.1021/ie303092j
Yadav M, Sarkar TK, Obot IB (2016) Carbohydrate compounds as green corrosion inhibitor: Electrochemical, XPS, DFT and molecular dynamics simulation studies. RSC Adv. https://doi.org/10.1039/C6RA24026G
Yadav M, Kumar S, Sinha RR, Behera D (2013) Experimental and quantum chemical studies on corrosion inhibition performance of benzimidazole derivatives for mild steel in HCl. Ind Eng Chem Res. https://doi.org/10.1021/ie400099q
Aslam R, Mobin M, Zehra S, Obot IB, Ebenso EE (2017) N, N′-dialkylcystine gemini and monomeric N-alkyl cysteine surfactants as corrosion inhibitors on mild steel corrosion in 1 M HCl solution: a comparative study. ACS Omega 2:5691–5707. https://doi.org/10.1021/acsomega.7b00501
Kumar SLA, Gopiraman M, Kumar MS, Sreekanth A (2011) 2-acetylpyridine-N(4)-morpholine thiosemicarbazone (HAcpMTSc) as a corrosion inhibitor on mild steel in HCl. Ind Eng Chem Res 50:7824–7832. https://doi.org/10.1021/ie200487g
Tawfik SM (2015) Alginate surfactant derivatives as ecofriendly corrosion inhibitor for carbon steel in acidic environment. RSC Adv. https://doi.org/10.1039/C5RA20340F
Hmamou DB, Salghi R, Zarrouk A, Aouad MR, Benali O, Zarrok H, Messali M, Hammouti B, Kabanda MM, Bouachrine M, Ebenso EE, Kabanda MM, Bouachrine M (2013) Weight loss, electrochemical, quantum chemical calculations and molecular dynamics simulation studies on 2-(benzylthio)-1,4,5-triphenyl-1Himidazole as inhibitor for carbon steel corrosion in hydrochloric acid. Ind Eng Chem Res. https://doi.org/10.1021/ie401034h
Oguzie EE, Li Y, Wang SG, Wang F (2011) Understanding corrosion inhibition mechanisms—experimental and theoretical approach. RSC Adv 1:866–873
Yadav M, Sinha RR, Kumar S, Sarkar TK (2015) Corrosion inhibition effect of spiropyrimidinethiones on mild steel in 15% HCl solution: insight from electrochemical and quantum studies. RSC Adv 5:70832–70848
Okafor PC, Liu CB, Zhu YJ, Zheng YG (2011) Corrosion and corrosion inhibition behavior of N80 and P110 carbon steels in CO2-saturated simulated formation water by Rosin Amide imidazoline. Ind Eng Chem Res 50:7273–7281. https://doi.org/10.1021/ie1024112
Quraishi MA, Sardar R (2002) Corrosion inhibition of mild steel in acid solutions by some aromatic oxadiazoles. Mater Chem Phys 78:425–431
Khaled KF, Abdel-Shafi NS (2014) Corrosion inhibition of mild steel by some sulfur containing compounds: artificial neural network modelling. J Mater Environ Sci 5(4):1288–1297
He C, Tian Z, Zhang B, Lin Y, Chen X, Wang M, Li F (2014) Inhibition effect of environment-friendly inhibitors on the corrosion of carbon steel in recirculating cooling water. Ind Eng Chem Res. https://doi.org/10.1021/ie504616z
Ahamad I, Quraishi MA (2009) Bis (benzimidazol-2-yl) disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros Sci 51:2006–2013
Quraishi MA, Gupta NK, Verma C, Mukherjee AK (2016) Green Schiff’s bases as corrosion inhibitors for mild steel in 1 M HCl solution: experimental and theoretical approach. RSC Adv. https://doi.org/10.1039/C6RA22116E
Ansari KR, Quraishi MA, Singh A (2017) Chromenopyridin derivatives as environmentally benign corrosion inhibitors for N80 steel in 15% HCl. J Assoc Arab Univ Basic Appl Sci 22:45–54
Eddy NO, Ebenso EE (2010a) Adsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic medium. Int J Electrochem Sci 5:731–750
Azzam EMS, Abd El-Aal AA (2013) Corrosion inhibition efficiency of synthesized poly 12-(3-amino phenoxy) dodecane-1-thiol surfactant assembled on silver nanoparticles. Egypt J Petrol. https://doi.org/10.1016/j.ejpe.2013.06.008
Li X, Deng S, Lin T, Xie X, Du G (2017) 2-Mercaptopyrimidine as an effective inhibitor for the corrosion of cold rolled steel in HNO3 solution. Case Stud Fire Safety. https://doi.org/10.1016/j.corsci.2017.02.011
Wang L (2001) Evalution of 2-Mercaptobenzamidazole as corrosion inhibition for mild steel in phosphoric acid. Corros Sci 43:2281–2289
Saha SK, Ghosh P, Hens A, Murmu NC, Banerjee PB (2015) Density functional theory and molecular dynamics simulation study on corrosion inhibition performance of mild steel by mercapto-quinoline Schiff base corrosion inhibitor. Phys E 66:332–341
Eddy NO, Ebenso EE (2010b) Quantum chemical studies on the inhibition potentials of some Penicillin compounds for the corrosion of mild steel in 0.1 M HCl. J Mol Model 16:1291–1306. https://doi.org/10.1007/s00894-009-0635-6
Fouda AS, Ellithy AS (2009) Inhibition effect of 4-phenylthiazole derivatives on corrosion of 304L stainless steel in HCl solution. Corros Sci 51:868–875
Gopiraman M, Selvakumaran N, Kesavan D, Kim IS, Karvembu R (2012) Chemical and physical interactions of 1-benzoyl-3,3-disubstituted thiourea derivatives on mild steel surface: corrosion inhibition in acidic media. Ind Eng Chem Res 51:7910–7922. https://doi.org/10.1021/ie300048t
Arslan T, Kandemirli F, Ebenso EE, Love I, Alemu H (2009) Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros Sci 51:35–47
Özcan M, Dehri İL (2004) Electrochemical and quantum chemical studies of some sulphur-containing organic compounds as inhibitors for the acid corrosion of mild steel. Prog Org Coat 51:181–187
Doner A, Solmaz R, Ozcan M, Kardas G (2011) Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci 53:2902–2913
Jafari H, Sayin K (2016) Sulfur containing compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Trans Indian Inst Met. https://doi.org/10.1007/s12666-015-0556-2
Azzam EMS, Hegazy MA, Kandil NG, Badawi AM, Sami RM (2015) The performance of hydrophobic and hydrophilic moieties in synthesized thiol cationic surfactants on corrosion inhibition of carbon steel in HCl. Egypt J Pet 24:493–503
Bouklah M, Hammoutia B, Aouniti A, Benhadda T (2004) Thiophene derivatives as effective inhibitors for the corrosion of steel in 0.5M H2SO4. Prog Org Coat 49:225–228
Ebenso EE, Isabirye DA, Eddy NO (2010) Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int J Mol Sci 11:2473–2498. https://doi.org/10.3390/ijms11062473
Torres VV, Rayol VA, Magalhães M, Viana GM, Aguiar LCS, Machado SP, Orofino H, D’Elia E (2014) Study of thioureas derivatives synthesized from a green route as corrosion inhibitors for mild steel in HCl solution. Corros Sci 79:108–118
Amar H, Braisaz T, Villemin D, Moreau B (2008) Thiomorpholin-4-ylmethyl-phosphonic acid and morpholin-4-methyl-phosphonic acid as corrosion inhibitors for carbon steel in natural seawater. Mater Chem Phys 110:1–6
Okafor PC, Liu X, Zheng YG (2009) Corrosion inhibition of mild steel by ethylamino imidazoline derivative in CO2-saturated solution. Corros Sci 51:761–768
Şahin M, Bilgic S, Yılmaz H (2002) The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl Surf Sci 195:1–7
Amin MA, Khaled KF, Mohsen Q, Arida HA (2010) A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros Sci 52:1684–1695
Weder N, Alberto RA, Koitz R (2016) Thiourea derivatives as potent inhibitors of aluminum corrosion: atomic-level insight into adsorption and inhibition mechanisms. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.5b11750
Khaled KF (2010b) Electrochemical investigation and modeling of corrosion inhibition of aluminium in molar nitric acid using some sulphur-containing amines. Corros Sci 52:2905–2916
Tamilarasan R, Sreekanth A (2013) Spectroscopic and DFT investigations on the corrosion inhibition behavior of tris(5-methyl-2-thioxo-1,3,4- thiadiazole)borate on high carbon steel and aluminium in HCl media. RSC Adv 3:23681–23691
Zor S, Saǧdinç S (2014) Experimental and theoretical study of sulfathiazole as environmentally friendly inhibitor on aluminium corrosion in NaCl Prot Metals. Phys Chem Surf 50(2):244–253
Sherif E-SM (2012) Effects of 3-amino-1,2,4-triazole-5-thiol on the inhibition of pure aluminum corrosion in aerated stagnant 3.5 wt% NaCl solution as a corrosion inhibitor. Int J Electrochem Sci 7(6):4847–4859
Sherif E-SM (2013) Electrochemical investigations on the corrosion inhibition of aluminum by 3-amino-1,2,4-triazole-5-thiol in naturally aerated stagnant seawater. J Ind Eng Chem 19(6):1884–1889
Lakshmi NV, Arivazhagan N et al (2013) The corrosion inhibition of aluminium in 3.5% NaCl by diisopropyl thiourea. Int J Chem Tech Res 5(4):1959–1963
Kaya S, Banerjee P, Saha SK, Tüzün B, Kaya C (2016) Theoretical evaluation of some benzotriazole and phospono derivatives as aluminum corrosion inhibitors: DFT and molecular dynamics, simulations approaches. RSC Adv. https://doi.org/10.1039/C6RA14548E
Tan Y, Srinivasan M, Pehkonen S, Chooi SY (2006) Effects of ring substituents on the protective properties of self-assembled benzenethiols on copper. Corros Sci 48:840–862
Ying-Cheng P, Ying W, Lu-Yuan X, Xiao-Yu G, Hai-Feng Y (2012) Adsorption behavior of methimazole monolayers on a copper surface and its corrosion inhibition. J Phys Chem C 116:3532–3538. https://doi.org/10.1021/jp2090318
Zor S (2014) Sulfathiazole as potential corrosion inhibitor for copper in 0.1 M NaCl. Prot Metals Phys Chem Surf 50:530–537
Appa Rao BV, Iqbal MY, Kumar KC, Reddy MN (2014) Corrosion protection of copper by self-assembled nano film of 4-amino-3-(octadecylthio)-6-methyl-1,2,4-triazinone. Indian J Chem Technol 21:188–198
Appa Rao BV, Iqbal MY, Sreedhar B (2010) Electrochemical and surface analytical studies of the self-assembled monolayer of 5-methoxy-2-(octadecylthio)benzimidazole in corrosion protection of copper. Electrochim Acta 55:620–631
Kuruvilla M, John S, Joseph A (2013) Electrochemical studies on the interaction of l-cysteine with metallic copper in sulfuric acid. Res Chem Intermed 39:3531–3543
Qin TT, Li J, Luo HQ, Li M, Li NB (2011b) Corrosion inhibition of copper by 2, 5 dimercapto-1, 3, 4-thiadiazole monolayer in acidic solution. Corros Sci 53:1072–1078
Rao BA, Iqbal MY, Reddy MN, Kumar KC (2014) Self-assembled nanofilm of 1, 2-dihydro-3-(octadecylthio) benzotriazine on copper for corrosion protection. Bull Mater Sci 37:185–197
Sherif E-SM, Erasmus R, Comins J (2007) Corrosion of copper in aerated acidic pickling solutions and its inhibition by 3-amino-1, 2, 4-triazole-5-thiol. J Colloid Interface Sci 306:96–104
Tansug G, Tuken T, Giray ES, Fındıkkıran G, Sıgırcık G, Demirkol O, Erbil M (2014) A new corrosion inhibitor for copper protection. Corros Sci 84:21–29
Mohamed S (2011) El-Deab, Interaction of cysteine and copper ions on the surface of iron: EIS, polarization and XPS study. Mater Chem Phys 129:223–227
Tüken T, Kıcır N, Elalan NT, Sığırcık G, Erbil M (2012) Self assembled film based on hexane-1, 6-diamine and 2-mercapto-ethanol on copper. Appl Surf Sci 258:6793–6799
Xue G, Huang X-Y, Dong J, Zhang J (1991) The formation of an effective anti-corrosion film on copper surfaces from 2-mercaptobenzimidazole solution. J Electroanal Chem Interfacial Electrochem 310:139–148
Zhang XH, Liao QQ, Nie KB, Zhao LL, Yang D, Yue ZW, Ge HH, Li YJ (2015) Self-assembled monolayers formed by ammonium pyrrolidine dithiocarbamate on copper surfaces in sodium chloride solution. Corros Sci 93:201–210
Zucchi F, Trabanelli G, Fonsati M (1996) Tetrazole derivatives as corrosion inhibitors for copper in chloride solutions. Corros Sci 38:2019–2029
Chen W, Hong S, Luo HQ, Li NB (2014) Inhibition effect of 2,4,6-trimercapto-1,3,5-triazine self-assembled monolayers on copper corrosion in NaCl solution. J Mater Eng Perform 23:527–537
Cheng Z, Mo S, Jia J, Feng J, Luo HQ, Li NB (2016) Experimental and theoretical studies of 4,6-diamino-2-mercaptopyrimidine as a copper inhibitor in 3.5 wt% NaCl solution. RSC Adv 6:15210–15219
Kilinççeker G, Demir H (2013) The inhibition effects of cysteine on the corrosion behaviour of copper in 3.5% NaCl solution. Anti-Corros Methods Mater 60:134–142
Rajkumar G, Sagunthala R, Sethuraman MG (2015) Investigation of inhibiting properties of self-assembled films of 4-aminothiophenol on copper in 3.5% NaCl. J Adhes Sci Technol 29:1107–1117
Rajkumar G, Sethuraman MG (2016) A study of copper corrosion inhibition by self-assembled films of 3-mercapto-1H-1,2,4-triazole. Res Chem Intermed 42:1809–1821
Sherif E, Park S-M (2006) 2-Amino-5-ethyl-1, 3, 4-thiadiazole as a corrosion inhibitor for copper in 3.0% NaCl solutions. Corros Sci 48:4065–4079
Badawy WA, Ismail KM, Fathi AM (2006) Corrosion control of Cu–Ni alloys in neutral chloride solutions by amino acids. Electrochim Acta 51:4182–4189
Dermaja A, Hajjaji N, Joiret S, Rahmounia K, Srhiri A, Takenouti H, Vivier V (2007) Electrochim Acta 52:4654–4662
Ramjia K, Cairns DR, Rajeswari S (2008) Appl Surf Sci 254:4483–4493
Kumari PDR, Nayak J et al (2011) 3-ethyl-4-amino-5-mercapto- 1,2,4-triazole as corrosion inhibitor for 6061-alloy in sodium hydroxide solution. Portugaliae Electrochim Acta 29(6):445–462
Balaskas AC, Curioni M et al (2015) Effectiveness of 2-mercaptobenzothiazole, 8-hydroxyquinoline and benzotriazole as corrosion inhibitors on AA 2024–T3 assessed by electrochemical methods. Surf Interface Anal 47(11):1029–1039
Kiani MA, Mousavi MF, Ghasemi S, Shamsipur M, Kazemi SH (2008) Inhibitory effect of some amino acids on corrosion of Pb–Ca–Sn alloy in sulfuric acid solution. Corros Sci 50:1035–1045
Abd El-Hafez GM, Badawy WA (2013) The use of cysteine, N-acetyl cysteine and methionine asenvironmentally friendly corrosion inhibitors for Cu–10Al–5Ni alloyin neutral chloride solution. Electrochim Acta 108:860–866
Srivastava V, Haque J, Verma C, Singh P, Lgaz H, Salghi R, Quraishi MA (2017) Amino acid based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: experimental, DFT and MD studies. J Mol Liq 244:340–352
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: Ashish Asatkar’s family name was corrected.
Rights and permissions
About this article
Cite this article
Verma, D.K., Dewangan, Y., Dewangan, A.K. et al. Heteroatom-Based Compounds as Sustainable Corrosion Inhibitors: An Overview. J Bio Tribo Corros 7, 15 (2021). https://doi.org/10.1007/s40735-020-00447-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00447-7