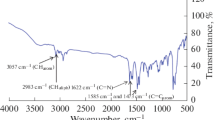
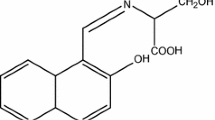
Abstract
The corrosion inhibition efficiency of newly synthesized Schiff bases, SB-1 [(4E)-N-((Z)-2-((furan-2-yl)methylimino) indolin-3-ylidene)(furan-2-yl)methanamine] and SB-2 [(7Z,8Z)-6-chloro-N2,N4-bis(1-(pyridin-2-yl)ethylidene)pyrimidine-2,4-diamine], was investigated for aluminium corrosion in 1 M H2SO4 medium using mass loss and electrochemical techniques. Potentiodynamic polarization curves show that addition of Schiff bases in the acid solution shifts the corrosion potential (Ecorr) towards positive direction, suggesting that chosen SBs are performed as mixed-type inhibitiors. The adsorption process of Schiff bases on aluminium surface obeys Langmuir isotherm. Further, electrochemical impedance studies (EIS) reveal that the inhibition efficiency remarkably rises with increasing SBs concentration and the maximum inhibition efficiencies of 97% and – 95% were obtained for SB-1 and SB-2, respectively, for the inhibitor concentration of 500 ppm. Additionally, the associated activation parameters and thermodynamic data of adsorption were evaluated. Scanning electron microscope (SEM) studies further confirm that ligands of SB-1 and SB-2 have a strong tendency to adhere on top of aluminium and protect its corrosion against acidic media.
Graphic Abstract
The SEM micrographs of corroded and inhibited aluminium surfaces and the maximum inhibition efficiency of aluminium corrosion are achieved to be 97% for Schiff bases (SB-1) in 1 M H2SO4 solution with the concentration of 500 ppm.













Similar content being viewed by others
References
Fouda AS, Al-Sarawy AA, Ahmed FS, El-Abbasy HM (2009) Corrosion inhibition of aluminium 6063 using some pharmaceutical compounds. Corros Sci 51:485–492
Nathiya RS, Raj V (2017) Evaluation of Dryopteris cochleata leaf extracts as green inhibitor for corrosion of aluminium in 1 M H2SO4. Egypt J Pet 26:313–323
Nathiya RS, Suresh P, Murugesan V, Anbarasan PM, Raj V (2017) Agarose as an efficient inhibitor for aluminium corrosion in acidic medium: an experimental and theoretical study. J Bio Tribo Corros 3:44
Nathiya RS, Perumal S, Murugesan V, Raj V (2018) Expired drugs: environmentally safe inhibitors for aluminium corrosion in 1 M H2SO4. J Bio Tribo Corros 4:4
Abiola OK, Otaigbe JOE (2008) Effect of common water contaminants on the corrosion of aluminium alloys in ethylene glycol–water solution. Corros Sci 50:242–247
He X, Jiang Y, Li C, Wang W, Hou B, Wu L (2014) Inhibition properties and adsorption behavior of imidazole and 2-phenyl-2-imidazoline on AA5052 in 1.0 M HCl solution. Corros Sci 83:124–136
El Nemr A, Moneer AA, Khaled A, El Sikaily A, El-Said GF (2014) Modeling of synergistic halide additives’ effect on the corrosion of aluminum in basic solution containing dye. Mater Chem Phys 144:139–154
Wang D, Li H, Liu J, Zhang D, Gao L, Tong L (2015) Evaluation of AA5052 alloy anode in alkaline electrolyte with organic rare-earth complex additives for aluminium-air batteries. J Power Sour 293:484–491
Zhu Y, Free ML, Yi G (2015) Electrochemical measurement, modeling, and prediction of corrosion inhibition efficiency of ternary mixtures of homologous surfactants in salt solution. Corros Sci 98:417–429
Abiola OK, Otaigbe JOE, Kio OJ (2009) Gossipium hirsutum L. extracts as green corrosion inhibitor for aluminum in NaOH solution. Corros Sci 51:1879–1881
Mercier D, Barthés-Labrousse MG (2009) The role of chelating agents on the corrosion mechanisms of aluminium in alkaline aqueous solutions. Corros Sci 51:339–348
Zhang J, Klasky M, Letellier BC (2009) Schiff bases of increasing complexity as mild steel corrosion inhibitors in 2 M HCl. J Nucl Mater 384:175–189
Shao HB, Wang JM, Zhang Z, Zhang JQ, Cao CN (2003) The cooperative effect of calcium ions and tartrate ions on the corrosion inhibition of pure aluminum in an alkaline solution. Mater Chem Phys 77:305–309
Lashgari M, Arshadi MR, Miandari S (2010) The enhancing power of iodide on corrosion prevention of mild steel in the presence of a synthetic-soluble Schiff base: electrochemical and surface analyses. Electrochim Acta 55:6058–6063
Küstü C, Emregül KC, Atakol O (2007) Schiff bases of increasing complexity as mild steel corrosion inhibitors in 2 M HCl. Corros Sci 49:2800–2814
Nathiya RS, Perumal S, Murugesan V, Raj V (2019) Evaluation of extracts of Borassus flabellifer dust as green inhibitors for aluminium corrosion in acidic media. Mater Sci Semicond Process 104:104674
Yıldız R (2015) An electrochemical and theoretical evaluation of 4,6-diamino-2-pyrimidinethiolas a corrosion inhibitor for mild steel in HCl solutions. Corros Sci 90:544–553
Zeng RC, Liu ZG, Zhang F, Li SQ, Cui HZ, Han EH (2014) Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. J Mater Chem A 2:13049–13057
Obot IB, Ebenso EE, Kabanda MM (2013) Metronidazole as environmentally safe corrosion inhibitor for mild steel in 0.5 M HCl: experimental and theoretical investigation. J Environ Chem Eng 1:431–439
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41
Mazumder MAJ, Al-Muallem HA, Faiz M, Ali SA (2014) Design and synthesis of a novel class of inhibitors for mild steel corrosion in acidic and carbon dioxide-saturated saline media. Corros Sci 87:187–198
Aytaç A, Özmen U, Kabasakaloğlu M (2005) Investigation of some Schiff bases as acidic corrosion of alloy AA3102. Mater Chem Phys 89:176–181
Li X, Deng S, Fu H, Xie X (2014) Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corros Sci 78:29–42
Oguzie EE, Oguzie KL, Akalezi CO, Udeze IO, Ogbulie JN, Njoku VO (2013) Natural products for materials protection: corrosion and microbial growth inhibition using Capsicum frutescens biomass extracts. ACS Sustain Chem Eng 2:214–225
Xhanari K, Finšgarv M, Hrnčič MK, Maver U, Knez Ž, Seiti B (2017) Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv 7:27299–27330
Xhanari K, Finšgar M (2016) Organic corrosion inhibitors for aluminium and its alloys in acid solutions: a review. RSC Adv 6:62833–62857
Safak S, Duran B, Yurt A, Türkoglu G (2012) Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros Sci 54:251–259
Gupta NK, Quraishi MA, Vermaa C, Mukherjee AK (2016) Green Schiff’s bases as corrosion inhibitors for mild steel in 1 M HCl solution: experimental and theoretical approach. RSC Adv 6:102076–102087
Gupta NK, Gopal CSA, Srivastava V, Quraishi MA (2017) Application of expired drugs in corrosion inhibition of mild steel. Int J Pharm Chem Anal 4(1):8–12
Abdel Hameed RS, Ismail EA, Abu-Nawwas AH, AL-Shafey HI (2015) Expired voltaren drugs as corrosion inhibitor for aluminium in hydrochloric acid. Int J Electrochem Sci 10:2098–2109
Garrigues L, Pebere N, Dabosi F (1996) An investigation of the corrosion inhibition of pure aluminum in neutral and acidic chloride solutions. Electrochim Acta 41:1209–1215
Totik Y, Sadeler R, Kaymaz I, Gavgali M (2004) The effect of homogenisation treatment on cold deformations of AA 2014 and AA 6063 alloys. J Mater Process Technol 147:60–64
Davis JR (1999) Corrosion of aluminium and aluminium alloys. ASM International, Ohio
Bai CY, Chou YH, Chao CL, Lee SJ, Ger MD (2008) Surface modifications of aluminum alloy 5052 for bipolar plates using an electroless deposition process. J Power Sour 183:174–181
Singh DK, Kumar S, Udayabhanu G, John RP (2016) 4(N,N-dimethylamino) benzaldehyde nicotinic hydrazone as corrosion inhibitor for mild steel in 1 M HCl solution: an experimental and theoretical study. J Mol Liq 216:738–746
Alaneme KK, Olusegun SJ, Adelowo OT (2016) Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions. Alexa Eng J 55:673–681
Fiori-Bimbi MV, Alvarez PE, Vaca H, Gervasi CA (2015) Corrosion inhibition of mild steel in HCl solution by pectin. Corros Sci 92:192–199
Asan A, Soylu S, Kıyak T, Yıldırım F, Öztas SG, Ancın N, Kabasakaloglu M (2006) Investigation on some Schiff bases as corrosion inhibitors for mild steel. Corros Sci 48:3933–3944
Njong RN, Ndosiri BN, Nfor EN, Offiong OE (2018) Corrosion inhibitory studies of novel Schiff bases derived from hydralazine hydrochloride on mild steel in acidic media. Open J Phys Chem 8:15–32
Yurt A, Duran B, Dal H (2014) An experimental and theoretical investigation on adsorption properties of some diphenolic Schiff bases as corrosion inhibitors at acidic solution/mild steel interface. Arab J Chem 7:732–740
Emregul KC, Atakol O (2003) Corrosion inhibition of mild steel with Schiff base compounds in 1 M HCl. Mater Chem Phys 82:188–193
Shetty Prakash (2019) Schiff bases: an overview of their corrosion inhibition activity in acid media against mild steel. Chem Eng Commun. https://doi.org/10.1080/00986445.2019.1630387
Chaitra TK, Mohana KN, Tandon HC (2018) Evaluation of newly synthesized hydrazones as mild steel corrosion inhibitors by adsorption, electrochemical, quantum chemical and morphological studies. Arab J Basic Appl Sci 25:1–11
Dohare P, Quraishi MA, Obot IB (2018) A combined electrochemical and theoretical study of pyridine based Schiff bases as novel corrosion inhibitors for mild steel in hydrochloric acid medium. J Chem Sci 130:1–19
Haque J, Srivastava V, Chauhan DS, Lgaz H, Quraishi MA (2018) Microwave-induced synthesis of chitosan Schiff bases and their application as novel and green corrosion inhibitors: experimental and theoretical approach. ACS Omega 3:5654–5668
Jamil DM, Al-Okbi AK, Al-Baghdadi SB, Al-Amiery AA, Kadhim A, Gaaz TS, Kadhum AAH, Mohamad AB (2018) Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem Cent J. https://doi.org/10.1186/s13065-018-0376-7
Singh DK, Ebenso EE, Singh MK, Behera D, Udayabhanu G, John RP (2018) Non-toxic Schiff bases as efficient corrosion inhibitors for mild steel in 1 M HCl: electrochemical, AFM, FE-SEM and theoretical studies. J Mol Liq 250:88–99
Tezcan F, Yerlikaya G, Mahmood A, Kardas G (2018) A novel thiophene Schiff base as an efficient corrosion inhibitor for mild steel in 1.0 M HCl: electrochemical and quantum chemical studies. J Mol Liq 269:398–406
Gomma GK, Wahdan MH (1995) Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater Chem Phys 39:209–213
Khaled KF, Hackerman N (2003) Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 0.5 M H2SO4 solutions. Mater Chem Phys 82:949
Musa AY, Mohamad AB, Kadhum AAH, Takriff MS, Tien LT (2011) Synergistic effect of potassium iodide with phthalazone on the corrosion inhibition of mild steel in 1.0 M HCl. Corros Sci 53:3672
Oguzie EE, Li Y, Wang FH (2007) Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. Corros Sci 310:90
Fouda AS, Mostafa HA, El-Taib F, Elewady GY (2005) Synergistic influence of iodide ions on the inhibition of corrosion of C-steel in sulphuric acid by some aliphatic amines. Corros Sci 47:1988
Dede B, Karipcin F, Arabal F, Cengiz M (2010) Synthesis, structure, and solvent-extraction properties of tridentate oxime ligands and their cobalt(II), nickel(II), copper(II), zinc(II) complexes. Chem Pap 64:25
Singh MK, Laskar R, Das A (2002) Synthesis and characterization of ionic heterobimetallic complexes of Ni(II), Cu(II), Zn(II) and Cd(II) ions containing nitrogen and sulphur donors. Ind J Chem Sec A 41:2282
Khoo J (2014) Synthesis, characterization and biological activity of two Schiff base ligands and their nickel(II), copper(II), zinc(II) and cadmium(II) complexes derived from S-4-picolyldithiocarbazate and X-ray crystal structure of cadmium(II) complex derived from pyridine-2-carboxaldehyde. Inorg Chim Acta 413:68
Silverstein RM, Webster FX, Kiemle DJ (2015) Spectrometric identification of organic compounds, 7th edn. Wiley, New York
Chaubey N, Singh VK, Quraishi MA (2017) Electrochemical approach of Kalmegh leaf extract on the corrosion behavior of aluminium alloy in alkaline solution. Inter J Indus Chem 8:75
Daoud D, Douadi T, Hamani H, Chafaa S, Noaimi MA (2015) Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: experimental and computational study. Corros Sci 94:21
Hany M, Lateef AE (2015) Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions. Corros Sci 92:104
Sanaulla PF, Lokesh HB, Murthy HCA, Raju B (2012) Electrochemical investigation of corrosion inhibition of AA6063 alloy in 1 M hydrochloric acid using Schiff base compounds. J Appl Chem 2:37
Bentiss F, Bouanis M, Mernari B, Traisnel M, Vezin H, Lagrenée M (2007) Understanding the adsorption of 4H-1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl Surf Sci 253:3696
Lebrini M, Lagrenée M, Traisnel M, Gengembre L, Vezin H, Bentiss F (2007) Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: electrochemical, X-ray photoelectron spectroscopy and theoretical studies. Appl Surf Sci 253:9267
Tao Z, Zhang S, Li W, Hou B (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51:2588
Zhang S, Tao Z, Li W, Hou B (2009) The effect of some triazole derivatives as inhibitors for the corrosion of mild steel in 1 M hydrochloric acid. Appl Surf Sci 255:6757
Oguzie EE, Njoku VO, Enenebeaku CK, Akalezi CO, Obic C (2008) Effect of hexamethyl pararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros Sci 50:3480
Doner A, Sahin EA, Kardas G, Serindag O (2013) Investigation of corrosion inhibition effect of 3-[(2-hydroxy-benzylidene)-amino]-2-thioxo-thiazolidin-4-one on corrosion of mild steel in the acidic medium. Corros Sci 66:278
Morad MS, Kamal El-Dean AM (2006) 2,2′-Dithiobis(3-cyano-4,6-dimethylpyridine): a new class of acid corrosion inhibitors for mild steel. Corros Sci 48:3398
Zhang QB, Hua YX (2009) Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim Acta 54:1881
Al Hamzi AH, Zarrok H, Zarrouk A, Salghi R, Hammouti B, Al-Deyab SS, Guenoun F (2013) The role of acridin-9(10H)-one in the inhibition of carbon steel corrosion: thermodynamic, electrochemical and DFT studies. Int J Electrochem Sci 8:2586–2605
Zhang B, He C, Wang C, Sun P, Li F, Lin Y (2015) Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water. Corros Sci 94:6
Heydari M, Javidi M (2012) Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52 steel in CO2-saturated solution and synergistic effect of iodide ions. Corros Sci 61:148
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nathiya, R.S., Perumal, S., Moorthy, M. et al. Synthesis, Characterization and Inhibition Performance of Schiff Bases for Aluminium Corrosion in 1 M H2SO4 Solution. J Bio Tribo Corros 6, 5 (2020). https://doi.org/10.1007/s40735-019-0291-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0291-z