Abstract
Moxifloxacin antibiotic investigated as green corrosion inhibitor for carbon steel in 1 M HCl solution using chemical and electrochemical methods in 1 M HCl solution at 25 °C. Results showed that Moxifloxacin is an efficient inhibitor for carbon steel and the efficiency reached to 94 % at 300 ppm. The inhibition efficiency increases with Moxifloxacin concentration and decreases with increasing temperature. The adsorption of Moxifloxacin on carbon steel surface follows Langmuir adsorption isotherm. The polarization plots revealed that Moxifloxacin acts as a mixed-type inhibitor. The impedance results showed that the charge transfer resistance will increase while, the double layer capacitance decreased by increasing the concentration of Moxifloxacin. The thermodynamic parameters of activation and adsorption processes were calculated and discussed. The surface morphology of the carbon steel specimens was analyzed using scanning electron microscope and energy dispersive X-ray analysis.
Similar content being viewed by others

1 Introduction
Evaluation of corrosion inhibitors for steel in acidic media signify for some industrial facilities as well as is very interesting from theoretical aspects. Acidic solutions are widely used in chemical laboratories and in several industrial processes such as acid pickling, acid cleaning, acid descaling, and oil well cleaning, etc. Also C-steel is utilized under different conditions in chemical and allied industries for handling alkaline, acid, and salt solutions [1]. Adsorbed organic compounds on metallic surface decrease the corrosion rate [2–4]. The adsorption observed depends mainly on certain physico-chemical properties of the inhibitor group, like functional groups, electron density at the donor atom, π-orbital character, and the electronic structure of the molecule [5–10].
Heterocyclic compounds such as antibiotic (pharmaceutical drugs) can provide excellent inhibition. These molecules depend at most on certain physical properties of the inhibitor molecules such as functional groups, steric factors, electron density at the donor atom, and electronic structure of the molecules [11]. A few researchers have reminded the use of antibacterial drugs as corrosion inhibitors because of the presence of oxygen, nitrogen, and sulfur in their structures as active centers, high solubility in water, high molecular size, nontoxic “environmentally friendly” corrosion inhibitors, important in biological reactions and drugs that can be easily produced and purified [12–19]. In recent years the use of drugs as corrosion inhibitors for different metals due to their nontoxic nature, namely Cefatrexyl, Ciprofloxacin, Norfloxacin, Ofloxacin drugs, and Tacrine have been paid attention on by some authors [20–22], therefore replacement by environmentally benign inhibitors is necessary. Recently, we have examined the inhibiting action of drugs such as Cefotaxime sodium, Cefazolin, Doxycycline, Pheniramine, Streptomycin, Cefalexin, Fexofenadine, Mebendazole, and Dapsone, on corrosion of metals in acid media [23–31]. The use of drugs as corrosion inhibitors is due to the presence of π electrons, heteroatoms in their molecules through which they are either adsorbed or form insoluble metal complex at the metal surface and inhibit metal corrosion [32].
Moxifloxacin drug is a fourth-generation synthetic fluoroquinolone antibacterial agent that has greater activity against Gram-(+) bacteria, anaerobes, and is nontoxic and available. Hence attempts are made to utilize this pharmaceutical drug as anticorrosion agent on carbon steel in HCl medium using chemical and electrochemical techniques. The surface morphology of the carbon steel specimens was evaluated using scanning electron microscope (SEM) and energy dispersive X-ray (EDX) analysis.
2 Experimental Techniques
2.1 Materials
Rectangular specimens with dimensions 2 × 2 × 0.2 cm of carbon steel with chemical composition seen in (Table 1) were used for all measurements. For electrochemical tests, the exposed surface area of the metal was 1 cm2.
2.2 Inhibitors and Chemicals
The pharmaceutical compound named Moxifloxacin has been investigated which was purchased from Memphis company, Cairo, Egypt. The investigated pharmaceutical drug is used as received and chosen because it is easily soluble in water, has high molecular weight, and contains many donating atoms (N, O, and P). All the solutions were prepared from AR grade chemicals using bidistilled water. The aggressive solutions used were made from 37 % HCl, appropriate concentrations of acid were prepared using bidistilled water. 1000 ppm stock solutions from the investigated inhibitor were prepared by dissolving 1 g/l of the solid pharmaceutical drug in bidistilled water; the other concentrations of pharmaceutical drug (50–300 ppm) were prepared by dilution with bidistilled water. All the materials used were of AR grade and used as received (Table 2).
2.3 Methods
2.3.1 Chemical Measurements
2.3.1.1 Weight Loss Measurements
Coupons of C-steel specimens of area equal to 8 cm2 were abraded with emery papers of 1/0, 2/0, 3/0, and 4/0 grade, degreased by acetone and weighed, then immersed in 100 ml of the test solution in open beakers. The beakers were placed into a water bath maintained at 25 °C. Each sample of C-steel was withdrawn from the test solution after every 30 min, washed, and dried in air before reweighing. The difference in weight after each interval was taken as the weight loss. The experiment was repeated at 25–45 °C. From the weight loss results, the inhibition efficiency (% IE) of the drug, degree of surface coverage (θ), and corrosion rate (k corr) were determined using the following equations [33]:
where W 1 and W 2 are the weight losses for C-steel in the presence and absence of the drug, A is the area of the C-steel coupon (cm2), t is the time of immersion (min), and W is the weight loss (mg cm−2) of C-steel after time t (min).
2.3.1.2 Hydrogen Evolution Method
Hydrogen Evolution Method assembly used for the measurement of hydrogen gas evolved from the corrosion reaction was described by Onuchukwu [34]. 100 ml of tested solution was used in each experiment. The time was recorded and H2 evolved was collected in a calibrated tube by displacement of water over time interval of 120 min. A plot of H2 evolved per unit area (ml/cm−2) against time (min) in the presence of different concentrations of the Moxifloxacin yield straight lines with slope that represent the rate of H2 evolved.
2.3.1.3 Thermometric Method
The reaction vessel and the procedure for determining the corrosion of carbon steel in 1 M HCl solution were the same as described before [35]. The test pieces were made of sheets measuring 1 × 10 × 0.2 cm. Before being used, these were lightly abraded with different grades of emery papers then degreased by acetone. Each experiment was carried out with a new clean specimen and with 15 mL of the aggressive solution. The variation of the temperature of the system was measured to ±0.1 °C on a calibrated thermometer.
The inhibiting efficiency was calculated from the percentage decrease in reaction number (RN) and was determined using the following equations:
The reaction number RN is defined as
where T m and T i are the maximum and initial temperatures, respectively and t is the time in minutes taken to reach T m.
2.3.2 Electrochemical Measurements
A three-electrode electrochemical cell was used. The working electrode was C-steel of surface area of 1 cm2. Before each experiment, the electrode was abraded using emery papers as before. After this, the electrode was cleaned ultrasonically with ethyl alcohol and washed by bidistilled water. All potentials were given with reference to the saturated calomel electrode (SCE). The counter electrode was a platinum plate of surface area of 1 cm2. The working electrode was immersed in the test solution during 30 min until a steady-state open circuit potential (E ocp) was obtained. The polarization curves were recorded by polarization from −0.6 to 0.2 V under potentiodynamic conditions corresponding to 1 mV/s (sweep rate) and under air atmosphere. All measurements were carried out with C-steel electrode in 1 M HCl in the absence and presence of different concentrations of the investigated drug at 25 °C. All experiments were carried out at 25 °C. The inhibition efficiency and surface coverage (θ) were calculated from the following equation:
where i corr (free) and i corr (inh) are the corrosion current densities in the absence and presence of inhibitor, respectively.
Electrochemical impedance spectroscopy (EIS) tests were performed using the same cell that was used in polarization experiments. The EIS was carried out over a frequency range of 1 Hz to 100 kHz, with a signal amplitude perturbation of 10 mV. The inhibition efficiency (% IE) and surface coverage (θ) of the investigated compounds obtained were calculated from the following equation:
where Rº ct and R ct are the charge transfer resistance values in the absence and presence of the inhibitor, respectively.
Electrochemical frequency modulation (EFM) is a nondestructive technique that can directly and rapidly give values of the corrosion current without a prior knowledge of Tafel constants. The great of the EFM is the causality factors, which serve as an internal check on the validity of the EFM measurement. With the causality factors the experimental EFM data can be verified [36]. Identical cell assembly was used in impedance studies. All electrochemical measurements were carried out using Potentiostat/Galvanostat/Zra analyzer (Gamry PCI4-G750). A personal computer with DC105 software for potentiodynamic, EIS300 software for EIS and EFM140 software for EMF and Echem Analyst v 5.21 was used for data fitting.
2.3.3 Surface Examinations
The specimens of C-steel used for surface morphology examination were immersed in 1 M HCl in the absence (blank) and presence of 300 ppm of Moxifloxacin at 25 °C for one day. The analysis was performed using scanning electron microscope (JEOL JSM-5500, Japan). Rough elemental analyses for the exposed surface were conducted by EDX technique.
3 Results and Discussion
3.1 Chemical Measurements
3.1.1 Weight Loss Measurements
Weight loss of carbon steel was determined, at various time intervals, in the absence and presence of different concentrations of Moxifloxacin. From the experimental data of the weight loss measurements, the inhibition efficiency % IE, was calculated from Eq. (1). All the experiments were performed at 25–45 °C. Values of corrosion rates (k corr) and % IE of Moxifloxacin are summarized in Table 3. The value of % IE increases with increasing drug concentration and decreases with rise in temperature. This behavior can be attributed to the increase of the surface coverage and due to the adsorption of drug on the surface of C-steel. The optimum concentration needed to achieve an efficiency of 94 % was found to be 300 ppm. In all cases, the increase in the inhibitor concentration was accompanied by a decrease in weight loss and an increase in the percentage inhibition. These results lead to the conclusion that the Moxifloxacin under investigation is fairly efficient as inhibitor for C-steel dissolution in HCl solution. The results confirmed the very good effect of Moxifloxacin on the corrosion inhibition of C-steel in 1 M HCl solution. Figure 1 shows the weight loss–time curves for the corrosion of C-steel in 1 M HCl solution in the absence and presence of Moxifloxacin at 25 °C.
3.1.1.1 Effect of Temperature
The effect of temperature on the corrosion parameters of C-steel with the addition of Moxifloxacin was studied using weight loss method. A major advantage of this method is its relative simplicity and availability. The data of corrosion behavior of C-steel in 1 M HCl containing different concentrations of Moxifloxacin for 120 min immersion in temperature range 25–45 °C are presented in (Table 4). Inspection of this Table reveals that the corrosion rate of C-steel increases with increased temperature. On the other hand, the inhibition efficiency of Moxifloxacin decreased with raising temperature Fig. 2. This suggests possible desorption of the adsorbed drug molecules from the metal surface at higher temperatures. Such behavior indicates that the additive was physically adsorbed on the metal surface.
Arrhenius-type dependence is observed between corrosion rate and temperature and often expressed as:
where \(E_{\text{a}}^{*}\) is the apparent activation energy, R is the universal gas constant, T is the absolute temperature, and A is the frequency factor. Figure 3 depicts Arrhenius plot log k corr against the reciprocal of temperature 1/T for C-steel in 1 M HCl solution in the absence and presence of different Moxifloxacin concentrations. An alternative formulation of Arrhenius equation is [37]:
where h is the Planck’s constant and N is the Avogadro’s number. Figure 4 shows a plot of log k corr/T as a function of 1/T for C-steel. Straight lines were obtained with a slope of −ΔH */R and an intercept of ln R/Nh + ΔS */R from which the values of ΔH * and ΔS * were calculated for the blank and Moxifloxacin. The values of the activation energy, enthalpy, and entropy are recorded in Table 5. The increase in the activation energy is proportional to the drug concentration, indicating that the energy barrier for corrosion process is also increased [38]. The increase in the activation enthalpy (ΔH *) in presence of the inhibitor means that the addition of the Moxifloxacin to the acid solution increases the height of the energy barrier of the corrosion reaction to an extent depends on the type and concentration of the present Moxifloxacin. The adsorption of the drug molecules on the metal surface leads to a lower number of hydrogen atoms adsorbed on it; this will cause a decrease in hydrogen evolution rate rather than the rate of metal dissolution, because of the blocking of the surface of the metal by the extract molecules.
3.1.1.2 Adsorption Isotherms
Attempts were made to fit the relationship between θ and the bulk concentration of the inhibitor used at a certain given temperature in order to give an insight into the adsorption process. Several adsorption isotherms are commonly used to characterize the drug performance and the best fit was obtained using Langmuir isotherm, which assumes that the solid surface contains a fixed number of adsorption sites and each site holds one adsorbed species [39] in good agreement with the following Eq. ( 9 ):
where K ads is the equilibrium constant of the adsorption process. A plot of (C/θ) versus (C) of Moxifloxacin at different temperatures present in Fig. 5 suggests that there are no attraction or repulsion forces between the adsorbed molecules, since a linear relationship is obtained with a slope equal to unity and intercept equal to 1/K ads. The adsorption equilibrium constant being related to the standard free energy of adsorption (\({{\Delta }}G_{\text{ads}}^{{{\circ }}}\)) by the relation ( 10 ):
where the constant 55.5 is the molar concentration of water in solution in M−1. By applying Eq. 6 , we obtain different values of \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) for drug as a function of temperature and are listed in Table 6. The results of this Table show the dependence of \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) on the absolute temperature (T). Figure 6 shows the relation between \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) and T. A linear plot was obtained with high regression constant and with a slope equal to the entropy of adsorption (\({{\Delta }}S_{\text{ads}}^{{{\circ }}}\)) and intercept equal to the enthalpy of adsorption (\(\Delta H_{\text{ads}}^{ \circ }\)), according to Eq. ( 11 ):
The various thermodynamic functions for the adsorption process are given in Table 6. It is generally obvious that \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) has negative value, which indicates that the tested drug is adsorbed spontaneously on C-steel surface forming a relatively stable adsorbed layer. It known that values of \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) lower than 20 kJ mol−1 is indicative of physical adsorption [40]. The value of \({{\Delta }}G_{\text{ads}}^{{{\circ }}}\) is approximately equal 23 kJ mol−1 which indicates that the adsorption mechanism of the Moxifloxacin on C-steel surface involves physical adsorption. The K ads follows the same trend in the sense that larger value of K ads means more efficient adsorption and hence better inhibition efficiency. The negative sign of \(\Delta H_{\text{ads}}^{{{\circ }}}\) reveals that adsorption of the inhibitor on C-steel surface from 1 M HCl solution is an exothermic process, which implies that % IE for the drug decreases with the rise in temperature. Such behavior can be explained on the basis that temperature rise causes desorption of some adsorbed drug molecules on the C-steel surface and hence lower protection was observed.
3.1.1.3 Hydrogen Evolution (HE) Tests
Data are plotted for the volume of H2 gas evolved against time in minutes for 50–300 ppm of Moxifloxacin concentrations and presented in Fig. 7. Slopes of such lines are estimated and taken as the rates of corrosion reaction as mentioned before. The good linearity in the relations indicates the presence of insoluble film on the metal surface during corrosion. The calculated corrosion rates (k corr) obtained from HE are tabulated in (Table 7). As observed, corrosion rate decreases with the increasing of Moxifloxacin concentration. According to Eq. (12) which pointed out that iron dissolution in HCl solutions depends on H+ ion more than the Cl− ion [41]. The hydrogen evolution and weight loss are produced by the same reaction:
Data are plotted for the volume of H2 gas evolved against time in minutes for 50–300 ppm of Moxifloxacin concentrations and presented in Fig. 7. Slopes of such lines are estimated and taken as the rates of corrosion reaction as mentioned before. The good linearity in the relations indicates the presence of insoluble film on the metal surface during corrosion.
3.1.1.4 Thermometric Measurements
In this method the temperature change was followed in the absence and presence of different concentrations of the Moxifloxacin as inhibitor. Curves of Fig. 8 represent the behavior observed in the presence of different concentrations of drug. All curves of the tested drug are characterized by an initial slow rise (due to the oxide film originally present on the metal surface) [42] followed by a sharp rise and finally by a decrease in temperature after attaining a maximum value. The curves for additive containing systems fall below that of the free acid. This indicates that the additive behaves as inhibitor over the concentration range studied. The percentage reduction in RN of the studied drug is presented in (Table 8). The results of (Table 8) reveal that the efficiency of corrosion inhibition as determined from the percentage reduction in RN varies with the concentration of the drug.
3.2 Electrochemical measurements
3.2.1 Potentiodynamic Polarization Tests
The polarization curves for C-steel in 1 M HCl solution with and without Moxifloxacin are presented in Fig. 9. Corrosion current densities were obtained from polarization curves by linear extrapolation of anodic and cathodic branches of Tafel slopes. The inhibition efficiency (% IE) and surface coverage (θ) were calculated from Eq. ( 5 ). The electrochemical parameters such as: corrosion potential (E corr), corrosion current density (i corr), anodic Tafel constant (b a), and cathodic Tafel constant (b c) are presented in Table 9. From the results, one can see when the concentration of Moxifloxacin was increased, the corrosion current density gradually decreased and the inhibition efficiency increased. The nearly steady values of (E corr) and the decreases of corresponding partial anodic and cathodic current densities, these data show that Moxifloxacin acts as mixed-type inhibitor [43]. Addition of Moxifloxacin has no significant effect on the values of anodic and cathodic Tafel slopes (b a, b c). Therefore, the addition of this drug blocked the active sites of the electrode surface, decreasing the surface area available for corrosion reaction [44]. Therefore the presence of various concentrations of Moxifloxacin does not alter the corrosion mechanism. The experimental findings of Tafel curves were in good agreement with the data obtained in case of weight loss.
3.2.2 EIS Tests
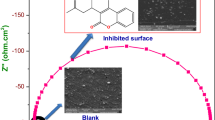
Figure 10 shows the Nyquist plot for C-steel in 1 M HCl in the absence and presence of different concentrations of Moxifloxacin at 25 °C. This diagram has a semicircle appearance; it indicates that the corrosion of Moxifloxacin is mainly controlled by a charge transfer process. The Bode plot for the C-steel is shown in Fig. 11 where the high frequency limit corresponds to electrolyte resistance R Ω, while the low frequency limit represents the sum of (R Ω + R p) where R p is the first approximation determined by both the electrolytic conductance of the oxide film and polarization resistance of the dissolution and passivation process. Various impedance parameter such as charge transfer resistance (R ct), double layer capacitance (C dl), and inhibition efficiency (% IE) were calculated and are given in Table 10. The data obtained show that the value of charge transfer resistance (R ct) increases while the value of double layer capacitance (C dl) decreases with increasing the concentration of the drug. This is due to the adsorption of the drug molecules on the electrode surface leading to a film formation of Moxifloxacin on C-steel surface. The obtained Nyquist diagram in most cases does not show perfect semicircle. This may be attributed to the frequency dispersion as a result of the inheterogeneity of the electrode surface [45, 46]. However, the diameter of the capacitive loop increases with the increasing concentration of Moxifloxacin.
3.2.3 EFM Tests
Figure 12 shows the EFM intermodulation spectra of carbon steel in HCl solution containing different concentrations of Moxifloxacin. The larger peaks were used to calculate the corrosion current density (i corr), the Tafel slopes (b c and b a), and the causality factors (CF-2 and CF-3). These electrochemical parameters are listed in Table 11. The data presented in Table 11 obviously showed that, the addition of Moxifloxacin at a given concentration to the acidic solution decreases the corrosion current density, indicating that this drug inhibits the corrosion of carbon steel in 1 M HCl through adsorption. The causality factors obtained under different experimental conditions are approximately equal to the theoretical values (two and three) indicating that the measured data are verified and of good quality. The inhibition efficiency (% IEEFM) increases by increasing the drug concentration and was calculated as from Eq. (5).
3.3 Surface Analysis
Figure 13a represents the surface of carbon steel without any addition. Figure 13 b gives the surface of C-steel after immersion in 1 M HCl solution, one can see the damage of the C-steel surface but Fig. 13c represents the surface of C-steel after the addition of 300 ppm drug. From this Figure, the surface is almost free from damages and it is smooth, this indicates a good protective film present on the carbon steel surface, and also confirms the highest inhibition efficiency of Moxifloxacin.
Figure 14 portrays the EDX analysis of C-steel in 1 M HCl only and in the presence of 300 ppm of the drug. The spectra show additional lines, demonstrating the existence of carbon (owing to the carbon atoms of the drug). These data show that the carbon and oxygen atoms covered the specimen surface. This layer is entirely owing to the inhibitor, because the carbon and oxygen signals are absent on the specimen surface exposed to uninhibited HCl. It is seen that, in addition to O, C was also present in the spectra. A comparable elemental distribution is shown in Table 12.
3.4 Mechanism of Corrosion Inhibition
The adsorption of drug molecules can be attributed to the existence of polar unit having atoms of nitrogen and oxygen and aromatic/heterocyclic rings. Therefore, the possible reaction centers are unshared electron pair of heteroatoms and л-electrons of the aromatic rings [47]. The adsorption and inhibition effect of drug molecules in 1 M HCl solution can be explained as follows: In aqueous acidic solutions, drug molecules exist either as neutral molecules or as protonated molecules and may adsorb on the metal/acid solution interface by one and/or more of the following ways: (i) electrostatic interaction of protonated molecules with already adsorbed chloride ions, (ii) interaction between unshared electron pairs of heteroatoms and vacant d-orbital of iron surface atoms. The possible explanation of the inhibition is due to the adsorption process which is considered as the key of the mechanism of inhibition action. It might be proposed that the drug molecules adhere to the steel surface. This leads to a decrease of the surface area at which cathodic and anodic reactions take place. Inhibition efficiency of the drug molecules depends on many factors [48], which include the number of adsorption active centers in the molecule and their charge density, molecular size, and mode of interaction with the metal surface [49, 50]. The transition of metal/solution interface from a state of active dissolution to the passive state of great interest. The inhibition effect by inhibitor is attributed to the adsorption of the inhibitor molecules via their functional group onto the metal surface, that drug can be adsorbed in the form of negatively charged species on the metal surface which can interact electrostatically with positively charged metal surface, leading to increase in the surface coverage, and consequently protect the efficiency in controlling the anodic metal dissolution and cathodic hydrogen evolution. The adsorption rate is usually rapid and hence the reactive metal is shielded from the aggressive environment.
4 Conclusions
The nontoxic drug of Moxifloxacin acts as a good and efficient inhibitor for the corrosion of C-steel in 1 M HCl medium. The maximum inhibition was found to increase with concentration and decrease with the raise in temperature. The adsorption of the drug on C-steel obeys Langmuir adsorption isotherm. A polarization study reveals that the drug acts via mixed mode of inhibition. The impedance method revealed that charge transfer process mainly controls the corrosion of C-steel. The SEM morphology of the adsorbed protective film on C-steel surface has confirmed the high performance of inhibitive effect of the Moxifloxacin. Results obtained from weight loss, hydrogen evolution, thermometric, DC polarization, AC impedance, and EFM techniques are reasonably in good agreement with each other as shown below:
Methods | Chemical | Electrochemical | ||||
|---|---|---|---|---|---|---|
Wt. loss | Thermometric | H E | EFM | EIS | Pot | |
% IE | 94.1 | 93 | 92.5 | 92.3 | 92 | 92 |
References
Bilgiç S (2002) The inhibition effects of benzoic acid and salicylic acid on the corrosion of steel in sulfuric acid medium. Mater Chem Phys 70:52–58
Bentiss F, Traisnel M, Lagrenée M (2000) The substituted 1, 3, 4-oxadiazoles: a new class of corrosion inhibitors of mild steel in acidic media. Corros Sci 42:127–146
Quraishi MA, Khan MAW, Ajmal M (1997) Influence of heterocyclic anils on corrosion inhibition and hydrogen permeation through mild steel in acid chloride environments. Corrosion 53:475–480
Khamis E (1990) The effect of temperature on the acidic dissolution of steel in the presence of inhibitors. Corrosion 46:476–484
Afidah A, Rahim E, Rocca J (2007) Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros Sci 49:402–417
Fouda AS, Al-Sharawy AA, El-Katori EE (2006) Pyrazolone derivatives as corrosion inhibitors for C-steel in hydrochloric acid solution. Desalination 201:1–13
Wang HL, Fan HB, Zheng JS (2002) Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater Chem Phys 77:655–661
Bhat JI, Alva VDP (2011) Inhibition effect of nevirapine an antiretroviral on the corrosion of mild steel under acidic condition. J Korean Chem Soc 55:835–841
Fouda AS, Elewady YA, Abd El-Aziz HK, Ahmed AM (2012) Corrosion inhibition of carbon steel in 0.5 M HCl solution using cationic surfactants. Int J Electrochem Sci 7:10456–10475
Fouda AS, El-desoky AM, Ead DM (2013) Anhydride derivatives as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int J Electrochem Sci 8:8823–8847
Fouda AS, Elmorsi MA, Fayed TA, Hassan AF, Soltan M (2014) Corrosion inhibitors based on antibiotic derivatives for protection of carbon steel corrosion in hydrochloric acid solutions. Int J Adv Res 2:788–807
Eddy NO, Stoyanov SR, Ebenso E (2010) Fluoroquinolones as corrosion inhibitors for carbon steel in acidic medium; experimental and theoretical studies. Int J Electrochem Sci 5:1127–1150
Samide A, Tutunaru B, Negrila C (2011) Corrosion inhibition of carbon steel in hydrochloric acid solution using a sulfa drug. Chem Biochem Eng 25:299–308
Mohan KN, Shivakumar SS, Badie AM (2011) Inhibition of carbon steel corrosion in 0.25 M sulphuric acid solution by ImatinibMesylate. J Korean Chem Soc 55:364–372
Von Fraunhofer JA, Stidham SH (1991) Effects of fused-ring antibiotics on metallic corrosion. Biochem Eng J 13:424–428
Solmaz R, Kardas G, Yazici B, Erbil M (2007) The rhodanine inhibition effect on the corrosion of carbon steel in acid along the exposure time. Prot Met 43:476–482
Fouda AS, Mostafa HA, El-Abbasy HM (2010) Antibacterial drugs as inhibitors for the corrosion of stainless steel type 304 in HCl solution. J Appl Electrochem 40:163–173
Abdallah M, Zaafarany I, Al-Fahemi JH, Abdallah Y, Fouda AS (2012) Antibacterial Cephalosporin as inhibitors for the corrosion of iron in hydrochloric acid solutions. Int J Electrochem Sci 7:6622–6637
Zhanga DQ, Cai QR, He XM, Gao LX, Kim GS (2009) Corrosion inhibition and adsorption behavior of methionine on copper in HCl and synergistic effect of zinc ions. Mater Chem Phys 114:612–617
Oguzie EE (2005) Corrosion inhibition of mild steel in hydrochloric acid solution by methylene blue dye. Mater Lett 59:1076–1079
Mylius FZ (1922) Der Aufbau der Zweistofflegierungen. EinekritischeZusammenfassung Metallkunde 14:233
Morad MS (2008) Inhibition of iron corrosion in acid solutions by Cefatrexyl: behaviors near and at the corrosion potential. Corros Sci 50:436–448
Xuehui P, Xiangbin R, Fei K, Jiandong X, Baorong H (2010) Inhibiting effect of ciprofloxacin, norfloxacin and ofloxacin on corrosion of mild steel in hydrochloric acid. Chinese J Chem Eng 18:337–345
Natarajaa SE, Venkateshaa TV, Tandonb HC (2012) Cetirizine: a new and effective corrosion inhibitor for mild steel in 1 M HCl solution. Corros Sci. 60:214–223
Shukla SK, Quraishi MA (2009) Cefotaxime sodium: a new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Corros Sci 51:1007–1011
Singh AK, Quraishi MA (2010) Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros Sci 52:152–160
Shukla SK, Quraishi MA (2010) The effects of pharmaceutically active compound doxycycline on the corrosion of mild steel in hydrochloric acid solution. Corros Sci 52:314–321
Ahamad I, Prasad R, Quraishi MA (2010) Inhibition of mild steel corrosion in acid solution by Pheniramine drug: experimental and theoretical study. Corros Sci 52:3033–3041
Shukla SK, Singh AK, Ahamad I, Quraishi MA (2009) Streptomycin: a commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater Lett 63:819–822
Shukla SK, Quraishi MA (2010) Cefalexin drug: a new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Mater Chem Phys 120:142–147
Ahamad I, Prasad R, Quraishi MA (2010) Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor. J Solid State Electrochem 14:2095–2105
Ahamad I, Quraishi MA (2010) Mebendazole: new and efficient corrosion inhibitor for mild steel in acid medium. Corros Sci 52:651–656
Singh A, Singh AK, Quraishi MA (2010) Aqueous extract of kalmegh (andrographispaniculata) leaves as green inhibitor for mild steel in hydrochloric acid solution. Open Electrochem J 2:41–51
Onuchukwu AI (1998) The kinetic and mechanism of hydrogen evolution on corroding Aluminium in alkaline medium. Master Chem Phys 25:235–277
Ahmed AI, Fouda AS, El-Askalany AE (1985) Inhinition of the corrosion of iron in nitric acid solution. Acta Chim Hung 120(1):57–62
Bosch RW, Hubrecht J, Bogaerts WF, Syrett BC (2001) Electrochemical frequency modulation: a new electrochemical technique for online corrosion monitoring. Corrosion 57:60–70
Abdel-Rehim SS, Khaled KF, Abd-Elshafi NS (2006) Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thiourea derivative. Electrochim Acta 51:3269–3277
Odiongenyi AO, Odoemelam SA, Eddy NO (2009) Corrosion inhibition and adsorption properties of ethanol extract of vernoniaamygdalina for the corrosion of mild steel in H2SO4. Port Electrochim Acta 27:33–45
Oguzie EE (2005) Inhibition of acid corrosion of mild steel by Telfariaoccidentalisextract. Pigm Resin Technol 34:321–326
Oguzie EE (2006) Studies on the inhibitive effect of Occimumviridis extract on the acid corrosion of mild steel. Mater Chem Phys 99:441–446
Okafor PC, Ebenso EE (2007) Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics. Pigm Resin Technol 36:134–140
Gad Allah AG, Nassif N, Mikhail T (1992) Effect of temperature on the corrosion behaviour of helwan steel in acid chloride solutions. Anal Chim 82:49–71
Kuhn AT, Shams El Din AM (1983) Thermometric and calorimetric methods in electrochemical and corrosion studies. Surf Technol 20:55–59
El Nemr A, Ghada FE, Khaled A, El Sikaily A, Abeer AM, Abd-El-Khalek DE (2014) Differences in the corrosion inhibition of water extracts of Cassia fistula L. pods and o-phenanthroline on steel in acidic solutions in the presence and absence of chloride ions. J Desalination Water Treat 52:5187–5198
Alsabagh AM, Migahed MA, Abdelraouf M, Khamis EA (2015) Utilization of green tea as environmentally friendly corrosion inhibitor for carbon steel in acidic media. Int J Electrochem Sci 10:1855–1872
Wang HL, Fan H, Zheng J (2003) Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater Chem Phys 77:655–661
Caigman GA, Metcalf SK, Holt EM (2000) Thiophene substituted dihydropyridines. J Chem Cryst 30:415–422
Singh AK, Quraishi MA (2010) Inhibitive effect of diethylcarbamazine on the corrosion of carbon steel in hydrochloric acid. Corros Sci 52:1529–1535
Avci G (2008) The Inhibition of Mild Steel Corrosion in 1 N HCl by ImidazoleDerivatives. Colloid Surf A 317:730–736
Fouda AS, Abdallah M, Ahmed IS, Eissa M (2014) Corrosion inhibition of aluminum in NaOH solutions using some bidentateazo dyes compounds and synergistic action with some metal ions. Int J Electrochem Sci 9:4747–4760
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fouda, A.S., Shalabi, K. & E-Hossiany, A. Moxifloxacin Antibiotic as Green Corrosion Inhibitor for Carbon Steel in 1 M HCl. J Bio Tribo Corros 2, 18 (2016). https://doi.org/10.1007/s40735-016-0048-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-016-0048-x