Abstract
Rice (Oryza sativa L.) grown on Zn mine waste contaminated soils has caused unequivocal Cd effects on kidney and occasional bone disease (itai-itai) in subsistence rice farmers, but high intake of Cd from other foods has not caused similar effects. Research has clarified two important topics about how Cd from mine waste contaminated rice soils has caused Cd disease: (1) bioaccumulation of soil Cd into rice grain without corresponding increase in Zn, and (2) subsistence rice diets potentiate Cd absorption/bioavailability and risk to farm families. Absorption of Cd by rice roots occurs on the NRAMP5 Mn2+ transporter. Although other transporters can influence Cd uptake-transport to shoots and grain, making NRAMP5 null greatly reduces grain Cd. Zn2+ has little ability to inhibit Cd2+ transfer in rice but clearly inhibits Cd uptake in other plant species. The bioavailability of dietary Cd is increased for subsistence rice diets. Research has identified that low levels and bioavailability of Zn and Fe in polished rice grain cause upregulation of Cd absorption on the Fe2+ transporter of duodenum cells (DMT1). Added dietary Zn can also inhibit intestinal Cd absorption somewhat. Nutritional stress (Fe, Zn deficiency) in humans consuming subsistence rice diets thus promotes Cd accumulation and adverse effects. No other dietary (crop) Cd exposure has caused unequivocal Cd-induced renal proximal tubular dysfunction (the first adverse Cd effect) in humans. Recognition of the very unusual nature of Cd risk from rice compared to other crops should be taken into account in setting international limits of Cd in rice and other foods.
Similar content being viewed by others
Introduction
Cadmium naturally occurs in Zn, Pb, and Cu ores with between 50 and 200 times more Zn than Cd on weight basis (Cd:Zn = 0.005–0.02 g Cd (g Zn)−1). One of the consequences of mining and smelting these ores has been the Cd contamination of surrounding soils which commonly occurred until recent times after environmental contamination was better understood. Cadmium contamination of soils has been a concern since it was determined that Zn mine wastes, containing high levels of Zn and Cd, had contaminated rice paddies and caused kidney and bone (itai-itai) diseases for subsistence rice farmers in the Jinzu Valley of Japan (see comprehensive review by Kobayashi [1]). The story of the discovery that Cd was the causal agent in itai-itai disease illustrates the unusual enrichment of Cd in diets of the subsistence rice farm families. The availability of a spectrograph which allowed measurement of Cd at low concentrations allowed analysis of soils, foods, and human tissues when few had previously studied Cd in biology. Subsequently, populations in many locations in Japan [2–5] and China [6, 7] and one location in Thailand [8] were similarly found to exhibit human Cd disease linked to subsistence rice farming although growing and smoking tobacco may have contributed to body Cd in some locations.
Cadmium in Zn mine waste contaminated soils is readily taken up by plants, is transported to different edible plant tissues, and thereby enters the food chain as a food contaminant [9, 10]. Food and smoking are the main pathways for human exposure to soil Cd. However, whether soil Cd reaches edible parts of plants is complex, varying in plant species and cultivars [11], and with soil properties especially soil pH, soil Eh, soil Zn, and chloride levels and Cd sorbent levels (Fe and Mn oxyhydoxides and organic matter) [12].
Cadmium in Soils and Plants
With a normal geochemical Cd/Zn ratio of 1:100 or greater, crops other than rice grown in contaminated soil have a natural limit to Cd uptake due to Zn phytotoxicity [13]; as the crop Cd level increases, the crop Zn level also increases. Most crops such as leafy vegetables, legumes, storage roots/tubers, fruits, and grains maintain low levels of Cd in edible tissues when Cd and Zn soil levels are at 1:100 Cd/Zn (0.01) ratio. When foliar Zn exceeds about 400–500 mg kg−1 dry weight (DW), Zn causes phytotoxicity reducing crop yields and causing growers to identify the cause of the visual symptoms (yellowing, or chlorosis due to Zn-induced Fe deficiency) [13], limiting the transfer of soil Cd to humans or animals [10]. Further, for crops other than rice, when foliar Zn is greatly increased to the point of Zn phytotoxicity, grain Zn is substantially increased [9]. Most Zn phytotoxicity occurs in strongly acidic soils which promote uptake of Cd and Zn by plants, and normal limestone addition to increase pH and improve crop yields reduces crop Cd and Zn levels.
Although human Cd disease has occurred with as little as 5 mg Cd kg−1 soil in rice soils, there have been cases where, as a result of vegetable garden soil Cd+Zn contamination, soil Cd levels were as high as 50–150 mg Cd kg−1, yet no kidney or bone diseases occurred in those consuming the produce, apparently because concurrent Zn levels were 5000–15,000 mg Zn kg−1 [10, 14–17]. However, while most rice paddies that are contaminated with Cd also have 100-fold higher Zn than Cd, this ratio did not provide the usual protection from Cd seen with other crops. Consumption of large amounts of seafood with Cd enrichment similarly had no effect on human Cd risk [18–20]. The interaction between Zn and Cd in rice is clearly an anomaly compared to other foods. In addition, Zn in test diets with food levels of Cd (not the high levels used by most toxicological researchers) limited increase of Cd in kidney and liver even when Cd was significantly increased compared to basal diets [21–25]. At food Cd levels, the co-contamination with Zn significantly reduces crop Cd bioavailability and risk except for rice consumption.
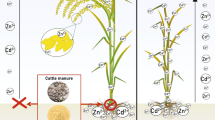
Cadmium accumulation by rice is anomalous compared to other staple crops. Takijima et al. [26, 27] found a wide range of Cd concentrations in rice paddy soils, yet found that total soil Cd was not correlated with Cd extractability nor with the Cd level in rice grain. Instead, flooding period and soil pH strongly affected rice grain Cd levels. In addition, the co-contamination with Zn had little influence on grain levels of Zn [28–30]. The paper by Fukushima et al. [31] on Cd and Zn in the rice consumed by the farm families at Jinzu Valley also reported that rice grain Cd could be sharply increased when soils were contaminated by Zn mine wastes, but grain Zn was not increased. In Thailand with extreme Cd+Zn contamination, grain Zn was not increased even when soils contained 7000 mg Zn kg−1 (Fig. 1) [32].
Zn in rice grain is not increased despite massive Zn mine waste contamination of rice soils with Cd and Zn, while grain Cd is strongly increased with any contamination (based on data in [32])
For rice culture, soil redox potential and pH control the amount of Cd in soil solution and Cd uptake by roots [26, 28, 30, 33]. Rice has traditionally been grown in flooded (anaerobic) soils. Under such anaerobic conditions, soil Mn and Fe oxyhydroxides are reduced to Mn2+ and Fe2+, and pH rises toward 7. Zn is either strongly adsorbed to soil sorbents or transformed to insoluble ZnS and Cd can precipitate as CdS [28, 34], although it has been difficult to document the extent of CdS formation in flooded rice soils even using Extended X-Ray Absorption Fine Structure (EXAFS) [35–37]. Although early researchers who reported the very strong effect of soil reduction on Cd uptake suggested that this could be explained by sulfide formation in flooded soils and formation of CdS [28], EXAFS evidence of CdS was first reported by Khaokew et al. [35] with highly contaminated Thai rice soils. De Livera et al. [36, 38] argue that these results did not explain the reduced Cd uptake adequately and suggested that mixed solid Cd-ZnS nanoparticles of sulfides undergoing redox might play a role. Subsequently, Fulda et al. [37] clearly showed that CdS was unequivocally formed in flooded Cd-spiked soils and that if sulfate was low compared to sulfide forming metals, or if Cu was high, CdS formation was appreciably inhibited and Cd uptake increased. Perhaps this finding explains the unusually high transfer of Cd to rice grain grown in soils contaminated by Cu mine wastes which caused human Cd disease at lower soil Cd than other locations [5, 39].
Now it is clear that the main reason that rice accumulates Cd during grain filling is the role of NRAMP5 at the same time that soil oxidation with great lowering of soil solution Mn, and soil acidification increases Cd solubility in the rhizosphere. Fulda et al. [37] reported that soluble Mn dropped remarkably within 2 days after their flooded soils with CdS were made aerobic, which would strongly reduce competition for Cd uptake on NRAMP5.
In common rice production, the paddies are drained at the beginning of flowering to promote higher yields, disease resistance, and convenience of harvest. As the drained paddy soils become aerobic and more acidic, CdS oxidizes, quickly becoming phytoavailable and causing rapid Cd uptake by rice (see [33, 40, 41]). A paper by Murakami et al. [42] testing phytoextraction of soil Cd using a Cd-accumulating cultivar of rice showed that while the soil remained flooded, rice shoot Cd did not exceed 0.5 mg kg−1 dry weight (DW), but after aerobic soil growth, shoots reached over 30–70 mg kg−1. Part of the increased phytoavailability of soil Cd is due to a strong reduction in soil pH in soils which occurs in non-calcareous rice soils as conditions become aerobic (e.g., [43]). The combination of these factors makes Cd more available for plant uptake without the inhibition by the higher levels of Zn, leading to a high uptake of Cd into the grain.
Because of the change in redox and soil pH during grain filling, soil extractions have been poor predictors of Cd accumulation in rice when either flooded or air-dried soils were tested. But, testing of field moist soils collected during grain filling for 0.1 M CaCl2-extractable Cd (1 g soil 5 mL−1) showed a strong correlation of extractable Cd with grain Cd [44] (Fig. 2). The equation for predicting Cd in brown rice based on 0.10 M CaCl2-extractable Cd of field-moist soil during the grain filling period was highly significant (Cd in brown rice = −0.007 + 3.32•Extr-Cd; R 2 = 0.95). Consuming such high Cd, low Zn and Fe rice grain caused human disease in many locations.
Correlation of Cd concentration in brown rice grain with Cd extractable from field-moist soils collected during grain filling using 0.1 M CaCl2 at 1 g soil (5 mL)−1 (based on data in [44])
Many studies of genetic variation in rice grain Cd have been reported. Some describe “pollution safe cultivars” [45], while others have identified the HMA3 genetic variation which when null so strongly increases Cd translocation to shoots and grain [46]. In most papers about “countermeasures” for contaminated rice fields, genetic improvement to lower Cd is included (e.g., [47]).
Experiments tested the ability of rice to accumulate solution Cd at different growth stages and to transport the Cd absorbed at several growth stages to the grain during filling. These studies showed that rice grown in aerobic nutrient solutions could absorb 109Cd and translocate it to leaves, and subsequently to grain at any time during growth [5, 29, 48]. Recently, Rodda et al. [49] studied Cd uptake and redistribution during grain filling and confirmed that part of the Cd absorbed into leaves during vegetative growth could move into grain but that most grain Cd was absorbed during the grain filling period and translocated directly to grain. As noted elsewhere, Cd is translocated from roots in xylem and transferred to phloem at shoot nodes and can be efficiently translocated to grain [50, 51]. Thus, it was not the ability of rice plants to only transport Cd to grain only during grain filling but that the uptake of Cd was greatly increased at the time of grain filling (due to aerobic soil conditions with lower pH, higher Cd2+, and lower Mn2+ in soil solution) that is the important factor in why rice accumulates Cd in grain so much more effectively than other cereal grains.
Recently, it was learned that Cd uptake by rice occurs largely on the NRAMP5 transporter, a Mn2+ transporter in rice roots [52–54]. Ishikawa et al. [52] generated heavy ion mutants of rice and tested Cd accumulation by 2592 M2 plants grown in Cd+Zn contaminated rice soils. They subsequently tested these lines in three contaminated fields in Japan, finding that the three lines had almost no Cd (undetected to 0.03 mg kg−1) accumulation in grain when the parent wild-type rice accumulated 0.55 to 1.8 mg Cd kg−1. Shoot Cd was similarly greatly reduced by the mutation.
During flooded culture, Mn2+ concentration in soil solution is greatly increased due to microbial reduction of soil MnO2, which inhibits Cd uptake on NRAMP5. Whether high soil solution Mn represses expression of NRAMP5 has not been clarified to date although the gene was reported to be constitutively expressed [54]. Levels of Mn2+ used in testing repression may not have been representative of reduced paddy soils. Further study of the interaction of Mn2+ with Cd2+ uptake by rice showed that soil solution levels of Mn2+ in flooded soils strongly inhibited Cd uptake [55] extending the findings of Fulda et al. [37] and Sasaki et al. [54]. Further, in contrast with Cd absorption by other plant species, increased Zn2+ had little effect on Cd absorption by NRAMP5. In previous studies of Cd uptake by wheat [56, 57], lettuce and spinach [58], and sunflower [59], increased activities of Zn2+ significantly reduced Cd accumulation to shoots when environmentally relevant Cd and Zn levels were used.
Environmentally relevant activities of microelement cations are stressed because in early study of microelement divalent cation uptake by pea, it was suggested that Cd uptake could occur on the IRT1 Fe2+ transporter of roots [60]. However, subsequent testing at other metal ion activities more similar to soil solution showed that IRT1 was only a Fe2+ transporter that likely played no role in accumulation of ions such as Zn2+, Cd2+, Ni2+, and Cu2+ [61]. Study of Zn uptake by rice has shown that there are at least two levels of uptake affinity, a high affinity transporter with a K m (concentration which gives half maximal uptake rate) of 0.010 to 0.020 μM and a low affinity transporter with K m of 6–20 μM [62]. Study at Cd levels >0.1 μM are irrelevant in understanding practical Zn accumulation from soils. Similar high affinity Zn transport activity was reported by Hacisalihoglu et al. [63] for bread wheat. Study at lower Cd2+ activity levels is difficult unless chelator buffering is used to control Cd and Zn activities at levels similar to soil solution. Green and Chaney (unpublished) showed that with Cd activity which gives rice Cd levels found in the field, increased Zn activity had little effect on Cd uptake, confirming the field observations that Zn does not inhibit Cd accumulation by rice. On the other hand, the “Chino” solution supplied more Mn and gave significantly lower Cd accumulation in rice shoots and grain than the “Grusak” solution both with the same buffered Cd2+ and Zn2 activity [64] (Table 1).
The high affinity Zn transporter in rice is believed to be ZIP1 which is expressed in the epidermal membrane of root cells [53, 65–68]. There have been many studies of Zn accumulation in rice in recent years because of the clear evidence that subsistence rice consumers may suffer Zn and Fe malnutrition (see [69]). Both agronomic and genetic methods to biofortify rice grain with Zn have been studied [70]. Improved bioavailable Zn in rice grain is also being evaluated for more water saving production methods (alternate wetting and drying). Even with aerobic soils, adding significant soluble Zn fertilizers has little effect on grain Zn concentration (e.g., [71]), while spray application of soluble Zn to leaves post-flowering (during grain filling) has significantly increased rice grain Zn levels [72, 73]. Stomph et al. [74] note the remarkable difference between rice and other studied grains in Zn uptake and transfer to grain in relation to soil applications. Further, although brown rice is increased appreciably in Zn by foliar Zn sprays during grain filling, endosperm is only slightly increased in Zn [75, 76].
The transition to more aerobic rice production (AWD) effect on rice grain Zn concentration and bioavailability are also complex [77, 78], while more aerobic soils invariably increase grain Cd (Fig. 3).
Effect of alternate wetting and drying flood management on accumulation of As and Cd in rice grain [79]. The water management varied from traditional full season flood, through alternative wetting and drying, to furrow irrigation only when the soil reached 40 or 60 % of water holding capacity
The interaction between metals in the uptake process can either be antagonistic (the addition of one decreasing the uptake of the other one) or synergistic (the addition of one increasing the uptake of the other). Antagonism between Cd and Zn has been reported by Honma and Hirata (see [29]) with rice, and Chaney et al. [59] with sunflower). Still other studies have reported synergism between Zn and Cd in rice, with an increase in Zn increasing the translocation of Cd from the roots to the shoots (see [29]). Yet when Cd and Zn were added in a ratio of 1:50 Cd:Zn, Cd concentration in the shoots decreased [29].
The demonstration of a key role of NRAMP5 in Cd accumulation in rice came from several research programs. In one, the researchers made mutants and grew out thousands of seeds to find any with lower accumulation of Cd in grain [52–54]. They characterized localization of the protein and expression, concluding that NRAMP5 was a significant transporter of Cd2+, Mn2+ and possibly Fe2+, but not Zn2+ into epidermal cells. Although in testing to date the NRAMP5 null rice genotypes maintain yields and grain quality, uptake of Mn is strongly reduced. If soils had not been flooded during the growth cycle in the research with the NRAMP5 null genotypes, it is possible that Mn would have become deficient for rice. Over a period of years of aerobic culture of low Mn soils such as long term rice soils which have undergone repeated reduction and oxidation over centuries, Mn phytoavailability can decline even to deficient levels (e.g., [80]). In that study, application of high rates of a high Fe biosolids to a low Mn Maryland soil caused Mn deficiency of wheat and soybean after about 15 years post biosolids application. It took that long for the soil redox and Mn chemistry to reach low phytoavailable Mn and cause Mn deficiency in wheat and even in maize. So it is not clear if the NRAMP5 null rice cultivar will maintain agronomic performance in the long term with aerobic production.
Long-term performance under aerobic conditions is an important question because of the newly understood need to produce rice aerobically to minimize accumulation of inorganic As (iAs) in rice grain. Because arsenate is reduced to the much more soluble (less strongly adsorbed) arsenite in flooded soils, and because arsenite is accumulated into rice on the silicate transporter [81], rice grown with traditional flood culture is higher in iAs than other food crops and may exceed the newly established CODEX limit of 0.20 mg iAs kg−1. Unfortunately, aerobic production to reduce grain iAs simultaneously causes grain Cd to increase as discussed above (see [40, 82] [Fig. 3]).
Other transporters are important in Cd accumulation in rice and other plant species. In particular, the HMA3 protein pumps Cd2+ into root vacuoles of rice [46, 83, 84] and soybean [85]. Over-expression of the HMA3 protein greatly reduced Cd transport to shoots and grain without changing uptake or transport of Zn or other nutrients [84]. However, adoption of transgenic improved cultivars over-expressing HMA3 is not accepted in many nations, and simple breeding using normal genotypes offers little hope of lowering crop Cd through change in HMA3 expression. HMA3-null rice has been identified as a potentially valuable phytoextraction crop because growing these genotypes under aerobic soil conditions, especially with acidic soils, allows high Cd accumulation in rice shoots, and the high shoot yields allow significant removal of Cd from the field [42, 86]. Another gene, HMA2, is involved in translocation of Cd and Zn from roots to shoots [87, 88], and yet another (LCT1) may affect low affinity Cd transport from leaves into grains [89].
An important advance in understanding of Cd uptake and translocation in rice was achieved by Fujimaki et al. [51] who used a positron-emitting tracer imaging system (PETIS) to follow Cd uptake and translocation in real time. This work illustrated the rapid transfer of Cd from xylem to phloem in basal nodes of the rice plant, helping to explain the highly efficient transfer of absorbed Cd into the grain after field drainage (see also [50]).
In other plant species, Zn strongly inhibits Cd uptake and translocation. The interaction can occur due to correction of Zn deficiency reducing upregulation of the Zn2+ transporter thereby reducing Cd uptake [90, 91], or the simple competition of Zn with Cd uptake by the ZIP1 discussed above.
Another important aspect of subsistence rice farming is that locally (home) grown crops are consumed by the farm family. For most other staple foods, crops are processed off-farm so that individuals do not consume only crops grown on contaminated soils for long periods. For urban populations, crops from many farms are commingled preventing consumption of only highly contaminated crop for long periods. Some other subsistence crops are consumed locally but are not known to accumulate bioavailable Cd as effectively as rice (maize, sorghum, bean, potato, casava) from common Cd+Zn mine or smelter contamination. It should be recognized that some Cd sources for soils lack the Zn of mine wastes, which allows high Cd phytoavailability and bioavailability thru all crops (e.g., [92]).
Absorption of Cd in Animal Intestine
Research using food levels of Cd has clearly shown that absorption of Cd is inhibited by Zn in the test meal [23–25, 93] and strongly increased by Fe deficiency status of the individual [94–96]. Research by Reeves and collaborators attempted to clarify the role of the nutrient deficiencies of rice subsistence diets in increased Cd absorption [97]. These studies showed that under marginal Fe-Zn-Ca malnutrition which did not reduce growth rates of rats, Cd absorption was increased as much as 10-fold compared to adequately nourished rats (not high levels) and that Cd in sunflower kernels had lower bioavailability than Cd in polished rice [98, 99]. They then did a pulse-chase kinetic study of 109Cd absorption and retention in tissues from Zn-Fe-Ca marginal diets versus adequate Zn-Fe-Ca diets which showed about 10-fold higher Cd absorption into enterocytes on the marginal diets, and long turnover in the intestine which caused much higher net Cd absorption (Fig. 4). After the dose, 109Cd continued to accumulate in the kidney until after the intestine had released all 109Cd. Further, a test with metallothionein-null mutant versus wild-type mice showed that metallothionein (a protein previously believed to play a significant role in Cd and Zn absorption into enterocytes) played no role in Cd absorption at dietary levels of Cd exposure in contrast with toxicological studies at high doses commonly reported [100]. This work illustrates the error in understanding animal Cd physiology which resulted from toxicological type testing with rats and other animals. In those studies, diet Cd is often higher than diet Zn or Fe, changing absorption patterns and competitions, and causing induction of metallothionein biosynthesis which traps Cd in the intestine cells. Huge numbers of papers on Cd absorption are irrelevant due to the use of toxicological doses rather than food Cd levels [10, 93, 97].
a Concentration of 109Cd in whole intestine of rats fed with Fe-Zn-Ca marginal or adequate diets during 64 days post-feeding; b concentration of 109Cd in kidney of rats fed with Fe-Zn-Ca marginal or adequate diets during 64 days post-feeding (based on [99])
Limits to Protect Humans from Cd in Contaminated Soils
Misunderstanding of food Cd risks has led to difficulty in setting limits for allowable Cd in foods. After years of review, the Joint Expert Committee on Contaminants and Food Additives (JECFA) [101] established 7 μg Cd (kg body weight)−1 week−1 as a limit for chronic ingestion of dietary Cd. A later European Food Safety Agency (EFSA) [102] examined the same data and suggested that maximum daily intakes of Cd should be no higher than 2.5 μg Cd (kg body weight)−1 week−1. The JECFA [103] panel re-evaluated the Cd limit and lowered it slightly and made the recommendation to use a monthly intake limit (25 rather than 30 μg Cd (kg body weight)−1 month−1) to stress the long-term nature of food Cd risks. Even with this international opinion, the EFSA panel continued with their lower Cd intake recommendation [104].
Based on the summary of dietary Cd exposure to Cd+Zn contaminated soils above, and the improved understanding of animal absorption of Cd at dietary levels, it seems clear that several important over-estimations of dietary Cd risk are being made by many scientists. If humans exposed to a number of food Cd contamination sources have been shown to consume the Cd but not have increased Cd in blood, kidney, or urine [19, 105], important differences in food Cd bioavailability are not being taken into account in derivation of food Cd limits. In addition, some foods have significantly lower Cd bioavailability due to the presence of oxalate, phytate, and fiber in the food. Spinach is a known Cd accumulator crop, but the presence of oxalate in this food substantially reduces net Cd absorption compared to other foods [23, 106] apparently due to formation of a Ca-Cd-oxalate co-precipitate in the digestive system. Numerous papers have illustrated that added Zn reduces absorption of Cd from test diets [24], even from intrinsic labelled diets [23, 97]. When crops are grown with geogenic ratios of Cd to Zn, Zn inhibits Cd uptake by the crop, limits yield of the crop with smaller increases in crop Cd, and also reduces Cd bioavailability in the edible crop to the consumer [107]. Because most Cd absorption research does not include the “natural” 100-fold increased crop Zn that accompanies Cd in mine waste contaminated soils and crops, predictions of risk from that source are greatly over-estimated for crops other than rice.
In addition, adverse effects of Cd in humans are often over-stated. Examination of the extensive human Cd exposure and disease in Japan suggests that no one would experience initial proximal renal tubular dysfunction until urinary Cd exceeded 10–12 μg (g creatinine)−1 in urine [108]. Examination of over 10,000 middle-aged non-smoking Japanese urban women consuming considerably higher daily Cd in their diet than Europeans (because of the background higher Cd in rice in Japan) showed that even when urine contained 3 μg Cd (g creatinine)−1, there was no evidence of renal tubular disease [109, 110] in direct contradiction of the views of the EFSA panel [102, 104]. One needs to recognize potential bias from selection of a cutoff of urinary β2-microglobulin or other proximal tubular dysfunction indicators in urine when the normal geometric mean is 100 and strong Cd disease causes β2-microglobulin to rise above 100,000 μg (g creatinine)−1 [111]. Further, when smoking is a more significant source of kidney Cd in smokers than food Cd for non-smokers, evaluating Cd effects in mixed populations of smokers and non-smokers may give artifacts. Many diseases are induced by chronic smoking, while chronic ingestion of Cd is only connected clearly with high consumption of rice homegrown on mine waste contaminated paddy soils which causes proximal tubular renal dysfunction and occasionally osteomalacia (itai-itai) after prolonged Cd kidney disease.
The new concerns about As in rice make Cd in rice a more important issue to be considered in management of rice production. As discussed above, growing rice with more aerobic soil management can significantly reduce iAs in rice grain, a desired outcome. However, any increase in aerobic condition during growth and especially during grain filling can significantly increase grain Cd (Fig. 3). Efforts to reduce rice iAs must balance this goal against the potential increase in rice Cd with more aerobic production practices.
References
Kobayashi J. Pollution by cadmium and the itai-itai disease in Japan. In: Oehme FW, editor. Toxicity of heavy metals in the environment. New York: Marcel Dekker, Inc; 1978. p. l99–260.
Nogawa K, Kobayashi E, Okubo Y, Suwazono Y. Environmental cadmium exposure, adverse effects and preventive measures in Japan. BioMetals. 2004;17:581–7.
Horiguchi H, Aoshima K, Oguma E, Sasaki S, Miyamoto K, Hosoi Y, et al. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int Arch Occup Environ Health. 2010;83:953–70.
Tsuchiya K. Cadmium studies in Japan: a review. New York: Elsevier/North-Holland Biomedical Press; 1978.
Asami T. Pollution of soils by cadmium. In: Nriagu JO, editor. Changing metal cycles and human health. Berlin: Springer-Verlag; 1984. p. 95–111.
Cai S, Yue L, Hu Z, Zhong X, Ye Z, Xu H, et al. Cadmium exposure and health effects among residents in an irrigation area with ore dressing wastewater. Sci Total Environ. 1990;90:67–73.
Jin T, Nordberg G, Ye T, Bo M, Wang H, Zhu G, et al. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ Res. 2004;96:353–9.
Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Padungtod C. Cadmium-exposed population in Mae Sot District, Tak Province: 1. Prevalence of high urinary cadmium levels in the adults. J Med Assoc Thail. 2007;90:143–8.
Chaney RL, Ryan JA, Li Y-M, Brown SL. Soil cadmium as a threat to human health. In: McLaughlin MJ, Singh BR, editors. Cadmium in soils and plants. Dordrecht: Kluwer Academic Publ.; 1999. p. 219–56.
Chaney RL, Ryan JA, Reeves PG. Cadmium in soils and its transfer to plants and the human food chain. In: Proc. Eighth International Cadmium Conference (Nov. 10–13, 2011, Kunming, China). Brussels, Belgium: International Cadmium Association; 2013. pp. 175–212.
Grant CA, Clarke JM, Duguid M, Chaney RL. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ. 2008;390:301–10.
Chaney RL. Cadmium and zinc (Chapter 17). In: Hooda P, editor. Trace elements in soils. Oxford: Blackwell Publ.; 2010. p. 409–39.
Chaney RL. Zinc phytotoxicity. In: Robson AD, editor. Proceedings of the international symposium on zinc in soils and plants. Dordrecht: Kluwer Academic Publisher; 1993. p. 135–50.
Chaney RL, Ryan JA. Risk based standards for arsenic, lead and cadmium in urban soils. (ISBN 3-926959-63-0). Frankfurt: DECHEMA; 1994.
Ewers U, Freier I, Turfeld M, Brockhaus A, Hofstetter I, König W, et al. Heavy metals in garden soil and vegetables from private gardens located in lead/zinc smelter area and exposure of gardeners to lead and cadmium (in German). Gesundheitswesen. 1993;55:318–25.
Sarasua SM, McGeehin MA, Stallings FL, Terracciano GJ, Amler RW, Logue JN, et al. Final Report. Technical Assistance to the Pennsylvania Department of Health. Biologic indicators of exposure to cadmium and lead. Palmerton: Part II. Atlants, GA: Agency for Toxic Substances and Disease Registry, US-DHHS; 1995. p. 1–57.
Strehlow CD, Barltrop D. The Shipham report – An investigation into cadmium concentrations and its implications for human health: 6. Health studies. Sci Total Environ. 1988;75:101–33.
Sharma RP, Kjellström T, McKenzie JM. Cadmium in blood and urine among smokers and non-smokers with high cadmium intake via food. Toxicology. 1983;29:163–71.
Vahter M, Berglund M, Nermell B, Åkesson A. Bioavailability of cadmium from shellfish and mixed diet in women. Toxicol Appl Pharmacol. 1996;136:332–41.
Sirot V, Samieri C, Volatier J-L, LeBlanc J-C. Cadmium dietary intake and biomarker data in French high seafood consumers. J Expo Sci Environ Epidemiol. 2008;28:400–9.
Chaney RL, Stoewsand GS, Bache CA, Lisk DJ. Cadmium deposition and hepatic microsomal induction in mice fed lettuce grown on municipal sludge-amended soil. J Agric Food Chem. 1978;26:992–4.
Chaney RL, Stoewsand GS, Furr AK, Bache CA, Lisk DJ. Elemental content of tissues of Guinea pigs fed Swiss chard grown on municipal sewage sludge-amended soil. J Agric Food Chem. 1978;26:944–97.
McKenna IM, Chaney RL, Tao SH, Leach Jr RM, Williams FM. Interactions of plant zinc and plant species on the bioavailability of plant cadmium to Japanese quail fed lettuce and spinach. Environ Res. 1992;57:73–87.
Jacobs RM, Jones AOL, Fry Jr BE, Fox MRS. Decreased long-term retention of 115mcadmium in Japanese quail produced by a combined supplement of zinc copper and manganese. J Nutr. 1978;108:901–10.
Fox MRS, Jacobs RM, Jones AOL, Fry Jr BE. Effects of nutritional factors on metabolism of dietary cadmium at levels similar to those of man. Environ Health Perspect. 1979;28:107–14.
Takijima Y, Katsumi F, Takezawa K. Cadmium contamination of soils and rice plants caused by zinc mining: 2. Soil conditions of contaminated paddy fields which influence heavy metal contents in rice. Soil Sci Plant Nutr. 1973;19:173–82.
Takijima Y, Katsumi F, Koizumi S. Cadmium contamination of soils and rice plants caused by zinc mining. 3. Effects of water management and applied organic manures on the control of Cd uptake by plants. Soil Sci Plant Nutr. 1973;19:183–93.
Iimura K. Chemical forms and behavior of heavy metals in soils. In: Kitagishi K, Yamane I, editors. Heavy metal pollution in soils of Japan. Tokyo: Japan Scientific Societies Press; 1981. p. 27–36.
Chino M. Uptake-transport of toxic metals in rice plants. In: Kitagishi K, Yamane I, editors. Heavy metal pollution in soils of Japan. Tokyo: Scientific Societies Press; 1981. p. 81–94.
Chino M. The assessment of various countermeasures against Cd pollution in rice grains. In: Kitagishi K, Yamane I, editors. Heavy metal pollution in soils of Japan. Tokyo: Scientific Societies Press; 1981. p. 281–5.
Fukushima M, Ishizaki A, Sakamoto M, Kobayashi E. Cadmium concentration in rice eaten by farmers in the Jinzu River basin (in Japanese). Jpn J Hyg. 1973;28:406–15.
Simmons RW, Pongsakul P, Chaney RL, Saiyasitpanich D, Klinphoklap S, Nobuntou W. The relative exclusion of zinc and iron from rice grain in relation to rice grain cadmium as compared to soybean: Implications for human health. Plant Soil. 2003;257:163–70.
Hattori H, Asari E, Chino M. Estimate of cadmium concentration in brown rice. Bangkok: 17th World Congress of Soil Science; 2002. p. 1992–1–5.
Ito H, Iimura K. Absorption of cadmium by rice plants in response to change of oxidation-reduction conditions of soils (in Japanese). J Sci Soil Manure Jpn. 1975;46:82–8.
Khaokaew S, Chaney RL, Landrot G, Ginder-Vogel M, Sparks DL. Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ Sci Technol. 2011;45:4249–55.
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG. Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ. 2011;409:1489–97.
Fulda B, Voegelin A, Kretzschmar R. Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ Sci Technol. 2013;47:12775–83.
de Livera J, McLaughlin MJ, Beak D, Hettiarachchi GM, Kirby J. Release of dissolved cadmium and sulfur nanoparticles from oxidizing sulfide minerals. Soil Sci Soc Am J. 2011;75:842–54.
Saito H, Shioji R, Hurukawa Y, Nagai K, Arikawa T, Saito T, et al. Cadmium-induced proximal tubular dysfunction in a cadmium-polluted area. Contrib Nephrol. 1977;6:1–12.
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S. Effect of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol. 2009;43:9361–7.
Kawasaki A, Arao T, Ishikawa S. Reducing cadmium content of rice grains by means of flooding and a few problems. Jpn J Hyg. 2012;67:478–83.
Murakami M, Nakagawa F, Ae N, Ito N, Arao T. Phytoextraction by rice capable of accumulating Cd at high levels: reduction of Cd content of rice grain. Environ Sci Technol. 2009;43:5878–83.
Chino M, Baba A. The effects of some environmental factors on the partitioning of zinc and cadmium between roots and tops of rice plants. J Plant Nutr. 1981;3:203–14.
Simmons RW, Noble AD, Pongsakul P, Sukreeyapongse O, Chinabut N. Analysis of field-moist Cd contaminated paddy soils during rice grain fill allows reliable prediction of grain Cd levels. Plant Soil. 2008;302:125–37.
Yu H, Wang J, Yuan J, Yang Z, Fang W. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ. 2006;370:302–9.
Ueno D, Koyama E, Yamaji N, Ma JF. Physiological, genetic, and molecular characterization of a high-Cd-accumulating rice cultivar. Jarjan J Exp Bot. 2011;62:2265–72.
Arao T, Ishikawa S, Murakami M, Abe K, Maejima Y, Makino T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010;8:247–57.
Chino M. The distribution of heavy metals in rice plants influenced by the time and the path of supply (in Japanese). J Sci Soil Manure Jpn. 1973;44:204–10.
Rodda MS, Li G, Reid RJ. The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil. 2011;347:105–14.
Yamaguchi N, Ishikawa S, Abe T, Baba K, Arao T, Terada Y. Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. J Exp Bot. 2012;63:2729–37.
Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, et al. Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010;152:1796–806.
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, et al. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci U S A. 2012;109:19166–71.
Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep. 2012;2:286.
Sasaki A, Yamaji N, Yokosho K, Ma JF. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell. 2012;24:2155–67.
Yang M, Zhang Y, Zhang L, Hu J, Zhang X, Lu K, et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J Exp Bot. 2014;65:4849–61.
Hart JJ, Welch RM, Norvell WA, Kochian LV. Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plant. 2002;116:73–8.
Hart JJ, Welch RM, Norvell WA, Clarke JM, Kochian LV. Zinc effects on cadmium accumulation and partitioning in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2005;167:391–401.
McKenna IM, Chaney RL, Williams FM. The effects of cadmium and zinc interactions on the accumulation and tissue distribution of zinc and cadmium in lettuce and spinach. Environ Pollut. 1993;79:113–20.
Chaney RL, Li Y-M, Schneiter AA, Green CE, Miller JF, Hopkins DG. Progress in developing technologies to produce low Cd concentration sunflower kernels. In: Proceedings of the 15th sunflower research workshop. Fargo: National Sunflower Association; 1993. p. 80–92.
Cohen CK, Fox TC, Garvin DF, Kochian LV. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 1998;116:1063–72.
Cohen CK, Garvin DF, Kochian LV. Kinetic properties of a micronutrient transporter from Pisum sativum indicate a primary function in Fe uptake from the soil. Planta. 2004;218:784–92.
Meng F, Liu D, Yang X, Shohag MJ, Yang J, Li T, et al. Zinc uptake kinetics in the low and high-affinity systems of two contrasting rice genotypes. J Plant Nutr Soil Sci. 2014;177:412–20.
Hacisalihoglu G, Hart JJ, Kochian LV. High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol. 2001;125:456–63.
Kukier U, Chaney RL. Growing rice grain with controlled Cd concentrations. J Plant Nutr. 2002;25:1793–820.
Ramesh SA, Shin R, Eide DJ, Schachtman DP. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 2003;133:126–34.
Yang X, Huang J, Jiang Y, Zhang H-S. Cloning and functional identification of two members of the ZIP (Zrt, Irt-like protein) gene family in rice (Oryza sativa L.). Mol Biol Rep. 2009;36:281–7.
Chen WR, Feng Y, Chao YE. Genomic analysis and expression pattern of OsZIP1, OsZIP3, and OsZIP4 in two rice (Oryza sativa L.) genotypes with different zinc efficiency. Russ J Plant Physiol. 2008;55:400–9.
Bashir K, Ishimaru Y, Nishizawa NK. Molecular mechanisms of zinc uptake and translocation in rice. Plant Soil. 2012;361:189–201.
Bouis HE, Welch RM. Biofortification – A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:S20–32.
Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302:1–17.
Phattarakul N, Rerkasem B, Li LJ, Wu LH, Zou CQ, Zhang FS, et al. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil. 2012;361:131–41.
Jiang W, Struik PC, Lingna J, van Keulen H, Ming Z, Stomph TJ. Uptake and distribution of root-applied or foliar-applied 65Zn after flowering in aerobic rice. Ann Appl Biol. 2007;150:383–91.
Boonchuay P, Cakmak I, Rerkasem B, Prom-U-Thai C. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci Plant Nutr. 2013;59:180–8.
Stomph TJ, Jiang W, Struik PC. Zinc biofortification of cereals: rice differs from wheat and barley. Trends Plant Sci. 2009;12:123–4.
Yoshikawa T, Kusaka S, Zikihara T, Yoshida T. Distribution of heavy metals in rice plants. I. Distribution of heavy metal elements in rice grains using an electron probe x-ray microanalyser (EPMA). J Soc Soil Manure Jpn. 1977;48:523–8. In Japanese.
Jiang W, Struik PC, van Keulen H, Zhao M, Jin LN, Stomph TJ. Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann Appl Biol. 2008;153:135–47.
Gao X, Hoffland E, Stomph T, Grant CA, Zou C, Zhang F. Improving zinc bioavailability in transition from flooded to aerobic rice. A review. Agron Sustain Dev. 2012;32:465–78.
Gao X, Zou C, Fan X, Zhang FS, Hoffland E. From flooded to aerobic conditions in rice cultivation: consequences for zinc uptake. Plant Soil. 2006;280:41–7.
Chaney RL, Anders MM, McClung A. Effect of Irrigation Water Management on As and Cd in Rice Grain. In Abstracts/Proceedings for International Plant Nutrition Colloquium, Istanbul, Turkey; 2013. pp. 182–83.
Brown SL, Angle JS, Chaney RL. Correction of limed-biosolid induced manganese deficiency on a long-term field experiment. J Environ Qual. 1997;26:1375–84.
Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105:9931–5.
Linquist BA, Anders M, Adviento-Borbe MA, Chaney RL, Nalley LL, da Rosa E, et al. Reducing greenhouse gas emissions, water use and grain arsenic levels in rice systems. Glob Chang Biol. 2015;21:407–17.
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011;189:190–9.
Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, et al. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci U S A. 2010;107:16500–5.
Benitez ER, Hajika M, TakahashI R. Single-base substitution in P1B-ATPase gene is associated with a major QTL for seed cadmium concentration in soybean. J Hered. 2012;103:278–86.
Ibaraki T, Fujitomi S-I, Ishitsuka A, Yanaka M. Phytoextraction by high-Cd-accumulating rice to reduce Cd in wheat grains grown in Cd-polluted fields. Soil Sci Plant Nutr. 2014;60:266–75.
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, et al. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012;35:1948–57.
Satoh-Nagasawa N, Mori M, Nakazawa N, Kawamoto T, Nagato Y, Sakurai K, et al. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012;53:213–24.
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, et al. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci U S A. 2011;108:20959–64.
Oliver DP, Hannam R, Tiller KG, Wilhelm N, Merry RH. The effects of zinc fertilization on cadmium concentration in wheat grain. J Environ Qual. 1994;23:705–11.
Chaney RL, Filcheva E, Green CE, Brown SL. Zn deficiency promotes Cd accumulation by lettuce from biosolids amended soils with high Cd:Zn ratio. J Resid Sci Technol. 2006;3:68–75.
Mench MJ, Martin E, Solda P. After effects of metals derived from a highly metal-polluted sludge on maize (Zea mays L.). Water Air Soil Pollut. 1994;75:277–91.
Fox MRS. Nutritional factors that may influence bioavailability of cadmium. J Environ Qual. 1988;17:175–80.
Flanagan PR, McLellan JS, Haist J, Cherian G, Chamberlain MJ, Valberg LS. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology. 1978;74:841–6.
Park JD, Cherrington NJ, Klaassen CD. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol Sci. 2002;68:288–94.
Ryu D-Y, Lee S-J, Park DW, Choi B-S, Klaassen CD, Park J-D. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett. 2004;152:19–25.
Reeves PG, Chaney RL. Bioavailability as an issue in risk assessment and management of food cadmium: a review. Sci Total Environ. 2008;398:13–9.
Reeves PG, Chaney RL. Mineral nutrient status of female rats affects the absorption and organ distribution of cadmium from sunflower kernels (Helianthus annuus L.). Environ Res. 2001;85:215–25.
Reeves PG, Chaney RL. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed a rice-based diet. Environ Res. 2004;96:311–22.
Reeves PG, Chaney RL, Simmons RW, Cherian MG. Metallothionein induction is not involved in cadmium accumulation in the duodenum of mice and rats fed diets containing high-cadmium-rice or sunflower kernels and a marginal supply of zinc, iron, and calcium. J Nutr. 2005;135:99–108.
JECFA. Evaluation of certain food additives and contaminants. Sixty-first Report of the Joint FAO/WHO Expert Committee on Food Additives. 2003. WHO Technical Report Series 922. http://whqlibdoc.who.int/trs/WHO_TRS_922.pdf.
EFSA (European Food Safety Agency). Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA J. 2009; 980:1–139. http://www.efsa.europa.eu/en/efsajournal/doc/980.pdf.
JECFA. Joint FAO/WHO Expert Committee on Food Additives, Summary Report on the 73rd meeting, (Geneva, 8–18 June, 2010). 2010. http://www.who.int/foodsafety/publications/chem/summary73.pdf.
EFSA (European Food Safety Agency). Statement on Tolerable Weekly Intake of Cadmium. EFSA J. 2011; 9(2):1975. http://www.efsa.europa.eu/en/efsajournal/doc/1975.pdf.
Reeves PG, Nielsen EJ, O’Brien-Nimens C, Vanderpool RA. Cadmium bioavailability from edible sunflower kernels: a long-term study with men and women volunteers. Environ Res. 2001;A87:81–91.
Buhler DR. Availability of cadmium from foods and water. In: Calabrese EJ, Tuthill RW, Condie L, editors. Inorganics in drinking water and cardiovascular disease. Princton: Princeton Scientific Publ. Co.; 1987. p. 271–87.
Chaney RL, Green CE, Ajwa H, Smith R. Zinc fertilization plus liming to reduce cadmium uptake by Romaine lettuce on Cd-mineralized Lockwood soil. Proc. Int. Plant Nutrition Colloquium XVI (Aug. 25–28, 2009, Sacramento, CA): 2009. Paper 1252. (http://repositories.cdlib.org/ipnc/xvi/1252).
Ikeda M, Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, et al. Threshold levels of urinary cadmium in relation to increases in urinary β2-microglobulin among general Japanese populations. Toxicol Lett. 2003;137:135–41.
Ezaki T, Tsukahara T, Moriguchi J, Furuki K, Fukui Y, Ukai H, et al. No clear-cut evidence for cadmium-induced renal tubular dysfunction among over 10,000 women in the Japanese general population: a nationwide large-scale survey. Int Arch Occup Environ Health. 2003;76:186–96
Ikeda M, Ezaki T, Moriguchi J, Fukui Y, Okamoto S, Ukai H, et al. No meaningful increase in urinary tubular dysfunction markers in a population with 3 μg cadmium/g creatinine in urine. Biol Trace Elem Res. 2006;113:35–44.
Tohyama C, Shaikh ZA, Nogawa K, Kobayashi E, Honda R. Elevated urinary excretion of metallothionein due to environmental cadmium exposure. Toxicology. 1981;20:289–97.
Acknowledgments
I gratefully acknowledge decades of discussion about rice and Cd with Dr. M. Chino, Dr. James A. Ryan, Dr. M.R.S. Fox, and Dr. Philip G. Reeves who all helped me sort out the soil, plant, and animal aspects of soil Cd risks to humans. Dr. Chino hosted my visit to Tokyo, Okayama (to visit Dr. Jun Kobayashi), Kanazawa (to visit Dr. K. Nogawa), Toyoma (to visit Dr. Hagino), and Tskuba in 1982 which helped me better understand the Jinzu Valley case and the program of soil remediation to reduce rice Cd levels in Japan, and to better design research to sort out factors involved and to seek improved understanding resulting in the present synthesis.
Conflict of Interest
The author has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Land Pollution
Rights and permissions
About this article
Cite this article
Chaney, R.L. How Does Contamination of Rice Soils with Cd and Zn Cause High Incidence of Human Cd Disease in Subsistence Rice Farmers. Curr Pollution Rep 1, 13–22 (2015). https://doi.org/10.1007/s40726-015-0002-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-015-0002-4