Abstract
Environmental variables influence Faecal Indicator Bacteria (FIB) in surface water. Understanding that influence is important, because presence of FIB, which are an indication of faecal contamination, means that harmful pathogens could be present that could also be influenced by environmental variables. Although some recent studies have focused on this topic, most of this work has been conducted in developed countries. Similar studies in developing countries and in a (sub)tropical climate are lacking. In this study we assess the influence of environmental variables on fluctuations in FIB concentrations of the Betna River in southwest Bangladesh that floods almost every year. Monthly water samples from five locations along Betna River were tested for FIB (E. coli and enterococci) in 2014–2015. A linear regression model was developed to assess the effect of the environmental variables on FIB concentrations. The study revealed increased FIB concentrations during wet weather conditions. Precipitation and water temperature were positively correlated with FIB concentrations. Water temperature was positively correlated, because the warm May to September period coincides with frequent precipitation. Precipitation increases manure release from land to surface water. The regression model explains nearly half of the variability in FIB concentrations (R 2 of 0.46 for E. coli and 0.48 for enterococci). This study indicates that increased precipitation combined with higher water temperature, as is expected in this region with climate change, likely increases FIB concentrations. Waterborne pathogens are expected to respond similarly to these environmental changes, indicating that disease outbreaks could well become more frequent and severe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to climate change, the frequency and intensity of extreme weather events, such as floods and cyclonic storm surges are increasing in Bangladesh (Ahmed et al. 2011). The effect of increased flooding is one of the major problems of Bangladesh. For example, over nine million people were affected by the 2008 flood events (NIDOS 2009). The southwest coastal area of Bangladesh is particularly vulnerable to the above extreme events. Vulnerability of coastal areas to flooding is aggravated by this area’s exposure to intense storm surges from the Bay of Bengal (CEGIS 2013). The recent devastating cyclones in 1988, 2002, 2007 and 2009 caused catastrophic flood in this region (ADB 2011).
Climate change induced heavy rainfall and flooding indeed has caused epidemics of waterborne diseases like diarrhoea (Delpla et al. 2009; Funari et al. 2012; Zhang et al. 2012). In Bangladesh, diarrhoeal disease remains very common, and every year it causes around 0.1 million deaths (Faruque 2014). These outbreaks of waterborne diseases are related to the concentration of waterborne pathogens in surface water (Freeman et al. 2009). The spread of waterborne pathogens and its relation to environmental variables is poorly studied (Rose et al. 2001; Patz et al. 2008; Hofstra 2011; Vermeulen and Hofstra 2013). In Bangladesh, increased concentrations of waterborne pathogens have been observed in the surface water after flooding events (Ahmed et al. 2010). An increase in polluted surface water containing high concentrations of pathogens with future climate change may increase the risk of waterborne diseases.
The water quality of surface waters has been deteriorating in developing countries due to rapid industrialization and population growth (Lata et al. 2016). The Betna River in southwest Bangladesh is a surface water source for about 0.1 million people living along the river. The river is contaminated by several sources of pollutants, such as untreated wastewater discharges, septic tank leakage, surface runoff from urban areas and agricultural lands, and manure storage areas. The untreated surface water is used for irrigation, domestic purposes and shellfish growing and this makes the population vulnerable to outbreaks of waterborne diseases.
The concentration of microorganisms in surface water may increase after extreme precipitation, because water may become contaminated by increased runoff, and resuspension from sediments (Hofstra 2011; Funari et al. 2012). Higher bacterial concentrations were reported during periods of intense precipitation and high river discharge (Schilling et al. 2009; Ibekwe et al. 2011; Aragonés et al. 2016). Conversely, increased precipitation may decrease the pathogen concentration of surface water due to dilution (Lucas et al. 2014). An increased temperature may cause die-off of pathogens and thereby reduce their concentrations (An et al. 2002; Walters et al. 2011; Vermeulen and Hofstra 2013). However, few studies reported positive correlation between water temperature and bacterial concentrations due to coincidence of summer temperature and periods of intense precipitation and high discharge (Koirala et al. 2008; Schilling et al. 2009). Some studies also reported the likelihood of some bacterial growth in tropical temperatures (Winfield and Groisman 2003; Tiefenthaler et al. 2009). However, the net contribution of environmental variables (temperature, precipitation and salinity) to changes in pathogen concentrations is not clear (Vermeulen and Hofstra 2013).
Surface water can be contaminated by a variety of pathogens, such as viruses, bacteria and parasites. The presence of faecal indicator bacteria (FIB) does not necessarily indicate the presence of pathogens (WHO 2008). The correlation between FIB and many waterborne pathogens is often weak. Nevertheless, microbial contamination of water bodies is usually detected by measuring the concentrations of FIB instead of pathogens (WHO 2008), because detecting varieties of pathogens is very difficult, time consuming and expensive (Bruhn and Wolfson 2007), whereas, measuring faecal indicators is easier, requires no complex equipment and is relatively cheap. It is therefore the most common method in use (Rochelle-Newall et al. 2015). Although FIB are generally not harmful themselves, their presence in water body indicates the possible presence of other pathogenic microorganisms (Burres 2009). Indicators are useful in assessing health risk, regardless of whether the specific pathogens are pathogenic or not (Wu et al. 2011; Teklehaimanot et al. 2014). E. coli, faecal coliforms and faecal streptococci have been most commonly used as microbial indicators of faecal contamination in water bodies. Enterococci are a subgroup within the faecal streptococcus group which have the ability to survive in salt water (Burres, 2009). Considering the high salinity level (i.e., up to 15 parts per thousand (ppt)) in the study area, we have used enterococci along with E. coli as an indicator of faecal contamination.
This paper assesses the influence of environmental variables (i.e., rainfall patterns, water temperature and salinity) on fluctuations in FIB concentrations of the Betna River in southwest Bangladesh that floods almost every year. First, we explore the spatial and temporal patterns of FIB variability and establish the correlation between observed environmental variables and concentration of FIB. Then, we estimate the relative contribution of these variables to the observed variation in FIB concentrations by fitting the data to a linear regression model. Finally, we discuss how environmental change influences FIB concentrations in a subtropical river system where this type of study is lacking (Rochelle-Newall et al. 2015). Therefore, the findings of this study will also be helpful for other developing countries with similar geographic setting.
2 Materials and Methods
2.1 Study Area
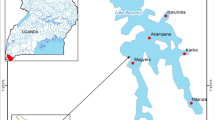
The study area covers an area of 107 km2 in the Betna watershed, located in the Satkhira district of southwest Bangladesh (Fig. 1). The total length of the river is about 192 km and its average width is 125 m. The Betna River is hydrologically linked with Bhairab River in the north and Kholpetua River near Assasuni in the south. The river flows from north to south and has tidal influence. The river has a number of small irrigation canals.
April to October is the wettest period with the highest rainfall intensity and November to February is the driest period with no or very little rainfall. Annual rainfall ranges from 1300 mm to 1900 mm with a 12 years average of 1640 mm (CEGIS 2013). More frequent and intense rainfall, with shorter rainfall periods have been observed over the last decades. Over the last five decades (i.e., 1948 to 2008), the cumulative rainfall has increased by 4.7 mm every 10 years (CEGIS 2013).
Two types of weather conditions cause floods. Firstly, during heavy rainfall in the monsoon, the excess water cannot be drained properly due to high siltation in the rivers and the canals. This results in water logging, which is also known as drainage congestion. Secondly, flooding occurs during cyclonic storm surges that emerge from the Bay of Bengal during the cyclone season in April–May (i.e., pre-monsoon). Flood hits the area almost every year (Hossain 2003; CEGIS 2013).
Non-calcareous grey floodplain soils are abundant in this area. The topsoil of the entire study area is clay (99%) and loam (1%). Agriculture is the dominant land use. About 61% of the study area is covered by farms for agriculture, 8% are settlements, 0.5% is forest, 10% are waterbodies and the remaining 20.5% is wetland (also used for aquaculture). In winter, due to the lack of upstream flow, salinity starts increasing and reaches up to 15 ppt in March–April. As a result agriculture is hindered in this season. During the rainy season from August–September the salinity reduces to nearly zero (IWM 2014).
The population density is 1050 people per km2 (BBS 2011). Many people reside and work on the river banks and their activities contaminate the Betna River. Sewage and manure are the main bacteria sources in this catchment. Wastewater is not treated and it is directly released into the river and canals (Kamal et al. 2008). During heavy rainfall, overflows of sewerage systems and septic tanks are common. The manure sources include manure applied to the agricultural farms as organic fertilizer, manure excreted from livestock grazing and direct deposition of animal faeces into the river and canals.
2.2 Sampling and Data Collection
To monitor faecal contamination of river water, water samples were collected from four locations along Betna River and one location from a nearby pond (Fig. 1). The sampling sites were selected to properly represent the various sewage sources (most importantly from the nearby town of Satkhira) and manure discharges into the river. Sampling sites 1 to 4 all receive microbial pollution from animal grazing and agricultural activities. The first sampling site (S1) was located in the upstream part of the study area. The river here occasionally receives overflow from a pond (i.e., Sampling site five) during heavy rainfall and/or flooding events. The second sampling site (S2) was located adjacent to some rural households. At the third sampling site (S3) the river receives sewage discharge from urban and industrial areas. Sampling site four (S4) was a connecting point of a creek that receives pollutants from Satkhira town. The fifth sampling site (S5; additionally incorporated after four months of sampling) represents a pond that directly receives human and household waste.
Water samples were collected once a month. These samples were generally taken between the 20th and 25th of every month and the sampling time was around 9.00 AM. Samples were collected for a period of 20 months from April 2014 to November 2015. A total of 96 samples were collected throughout the 20 months (except for S5, where sampling was only done for the last 16 months). Water temperature (instrument name: TLX, Dth-73), salinity (Hanna HI 8033), Electrical Conductivity (EC) (Hanna HI 8633), pH (Hanna HI 2211) and turbidity (Hach 2100Q) were recorded on each site at the time of sampling, while bacterial measurements were done later in the Environmental Microbiology Laboratory of Environmental Science Discipline, Khulna University, approximately 45 km from the sampling sites. Rainfall (total mm/day) data were collected from Bangladesh Water Development Board’s nearest station at Benarpota (see Fig. 1).
2.3 FIB Analysis
Water samples from the selected sites were collected at a depth of 50 cm from one river bank into sterile nalgene plastic bottles facing the mouths of the bottles upstream. All samples were collected with the care required for FIB analysis. Sampling bottles were sterilized using procedures described in standard methods (APHA 1992). All samples were placed in an insulated box filled with ice packs, transported to the laboratory and the analyses were started within six hours of collecting the first sample.
Enumeration of E. coli and enterococci were performed by the membrane filtration (MF) technique as described by USEPA (2002), Method 1103.1 and USEPA (2009), Method 1106.1 respectively. Several dilutions of samples were considered. We considered triplicate plates for each dilution to determine the number of bacteria. Samples were diluted with deionized water to an end volume of 100 mL. Each of the diluted samples was filtered through 0.45 μm membrane filter (Millipore Corp., Bedford, MA, USA). Filtration devices were treated by using a burner to ensure proper sterilization and to prevent cross contamination among samples. For enumeration of E. coli, the mTEC agar plates were incubated at 35 ± 0.5 °C for two hours followed by further incubation at 44.5 ± 0.2 °C for 22–24 h. Then, the filters were transferred to a pad saturated with urea substrate for 15 to 20 min. After incubation on the urea substrate at room temperature, yellow, yellow-green, or yellow-brown colonies were counted as E. coli. For enumeration of enterococci, mE agar plates were incubated at 41 ± 0.5 °C for 48 h followed by incubation on Esculin Iron Agar (EIA) plate for 20 to 30 min at 41 ± 0.5 °C. After incubation on the EIA black or reddish-brown colonies were counted as enterococci. The bacteria colonies were expressed as colony forming units (cfu) per 100 mL.
2.4 Statistical Analysis
All the data were analysed using the statistical package software SPSS 22.0. First, FIB ( E. coli and enterococci) observational data were log10 transformed to achieve a normal distribution. Normality of datasets was confirmed using Q-Q plots. The log10 transformed FIB data were always used in all statistical analyses. Water temperature and salinity data were normally distributed. However, precipitation data were gamma distributed, because the precipitation data contained many zero values. Precipitation often requires some time to flush manure into the river throughout the catchment. We analysed FIB data of three heavy rainfall events and found that FIB concentrations remain high in the river water until three days after the rainfall event (data not presented). We, therefore, summed the precipitation data over three days. Such precipitation summation is commonly used (Crowther et al. 2001; Walters et al. 2011; Vermeulen and Hofstra 2013).
A standard Pearson product–moment correlation analysis was performed for correlations between log10 transformed FIB concentrations and water physico-chemical parameters (temperature, salinity, pH, EC and turbidity). Correlation analysis between log10 FIB concentrations and precipitation was performed using the Spearman’s rank correlation, which does not require normally distributed data.
To assess the relative contributions of environmental variables to the observed difference in FIB levels in river water, the data were fitted to a linear regression model. Bacterial concentrations were used as dependent variables and as independent variables initially we had included water temperature, precipitation, and salinity. Other observed parameters (pH, EC and turbidity) did not show any significant correlation with FIB concentrations and were not included in the model.
The models of the following form were applied for each FIB:
where, Y is the FIB concentration in cfu per 100 mL, averaged over the four river locations, β i are constants, t is the water temperature in oC, p is the precipitation summed over 3 days, s is salinity in ppt, and ɛ is residual error. At the outset of modelling, collinearity among variables was examined and none was found that can violate the multicollinearity assumption. After running the model with the included variables, we studied the influence of these variables on concentrations of E. coli and enterococci. We eliminated the variable from the final model that had no significant influences on bacterial variability. We checked for interaction effects among variables, and no interaction effect was found significant. To test the sensitivity of the model and to assess the contribution of individual variable to the model outcome, we also ran the model leaving each of the independent variables out of the model. One-way analyses of variance (ANOVA) was performed to compare data sets and to assess the relative contribution of different variables to the observed variations in FIB concentration. The coefficient of determination (R 2), adjusted for degrees of freedom, was used to measure the proportion of the variability in FIB concentrations that is explained by the independent variables. All statistical tests were considered significant at a confidence level of 95% (p < 0.05).
The sampling sites S2, S3 and S4 that are situated closest to each other were found to have similar FIB concentrations (see Table 1), i.e., they are highly correlated. Spatial autocorrelation or spatial dependency occurs when the values of variables measured at nearby sites are not independent from each other (Tobler 1970). This implies that the independence assumption of the data is not fulfilled, and p-values can be highly underestimated. Because of the spatial correlation among sampling sites, the regression model was run for mean FIB concentrations over the four river sampling points. We do not expect to find time-dependent correlation in our measurements, as they were usually taken once a month. However, to conform that indeed no temporal autocorrelation exists, a Durbin-Watson test (Montgomery et al. 2001) was performed and no such correlation was found. Therefore, this method is appropriate for this analysis.
3 Results
3.1 Spatiotemporal Variations
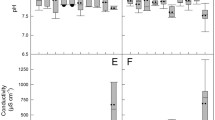
The measured water temperatures were between 22 and 32 °C. Precipitation occurred on three days preceding the sampling day in half of all FIB measurement days. Maximum 3-day precipitation was 116 mm, occurring on the 7th to 9th of July 2015. The lowest salinity was 0.1 ppt observed during the rainy season in the month of August, and highest was 12.7 ppt in May, 2015 (Fig. 2).
Concentrations of FIB vary substantially in time and space (Table 1, Fig. 2). Comparatively higher concentrations of FIB were found at S1, located upstream. This site receives pollutants from both municipal and agricultural sources. However, there was no statistically significant difference of FIB concentrations among the river sampling sites. S5 (the pond) had the highest concentration in all months and seasons.
FIB concentrations showed a clear seasonality with higher mean concentrations occurring during wet weather in the monsoon (July to October) of 2014–2015 and during storm surges (May and June) of 2014 (Fig. 2). Mean FIB concentrations in the wet weather were one to two orders of magnitude higher compared to those of dry weather (October to March). This difference was statistically significant. Wet weather was defined as rainfall larger than 5 mm/day within a week preceding the sampling day. Consequently, dry weather is defined as <5 mm in a week previous to the sampling day. The result also revealed that high FIB levels do not necessarily depend on the amount of rainfall. Even with little rainfall (<10 mm), FIB concentrations are high and can rapidly increase (Fig. 2). High E. coli concentration of 2.9 × 104 colony-forming units (cfu)/100 mL were found in S1 during June after a 7.4 mm rainfall event. However, highest concentrations of E. coli (3.6 × 104 cfu/100 mL) and enterococci (8.6 × 104 cfu/100 mL) were found in the pond (S5) during August 2015 after a heavy rainfall event of 88.4 mm.
The enterococci concentrations were not always consistent with the E. coli concentrations. Comparatively higher enterococci than E. coli concentrations were observed in most cases. The variation of concentrations between the two bacteria was found statistically significant across sites. The measured mean concentrations were between 2.9 and 3.4 log cfu/100 mL for E. coli and 3.5 and 4.0 log cfu/100 mL for enterococci (Table 1). Approximately 88% of the E. coli samples exceeded USEPA daily (single sample) bathing water quality standards of 235 cfu/100 mL. For enterococci, all samples exceeded the daily threshold of 104 cfu/100 mL. E. coli samples that did not exceed the standards were measured during dry winter months (November to April).
3.2 Correlations
Table 2 presents the correlations of different environmental variables with FIB measured at all sampling sites. Correlation analysis showed that the FIB levels were significantly linked with environmental variables: water temperature, precipitation, and salinity. Fig. 3 graphically shows the correlation between water temperature, precipitation, and salinity with FIB concentrations of all individual river sites. Log10 FIB was significantly correlated with precipitation in all individual measurement sites. R values range from 0.57 to 0.62 for E. coli and 0.50 to 0.71 for enterococci. Non-significant positive correlation was found between E. coli and water temperature in all the river sites, while in S5 (which is a pond nearby the river), the correlation was significantly positive. In case of enterococci, two (S1 and S2) out of the five sites showed significant positive correlation with water temperature, while in the other three sites the positive correlation was not statistically significant. Correlation between water salinity and FIB were all negative with one site for E. coli (S5) and two sites (S4 and S5) for enterococci having significantly negative correlations. FIB concentrations did not significantly correlate with other parameters studied (pH, EC and turbidity). Heavy rainfall and warmer water temperatures were found to have the strongest correlation with FIB concentrations in the surface waters.
3.3 Statistical Modelling
A linear regression model was developed to assess the combined effect of the environmental variables on FIB concentration in river water. The model, which is described in Section 2.5 gave an adjusted R 2 value of 0.46 for E. coli and 0.48 for enterococci (Table 3). Table 3 presents the β coefficients that indicate how much the dependent variable (mean FIB concentrations) varies with an independent variable while other independent variables remain constant. The regression analysis showed that two independent variables, water temperature and precipitation contributed significantly to the variations in mean FIB concentrations.
The correlation analysis revealed that salinity has a strong negative correlation for some sampling sites, but including salinity did not improve the model. That means that salinity contributed little to the model. We also ran the model with precipitation and salinity excluding temperature. This time the resulted adjusted R 2 values were 0.36 for E. coli and 0.23 for enterococci. This is low compared to the result of the previous model, meaning that water temperature contributes more to the variability in surface water FIB concentrations than salinity. This is also consistent with the correlation analysis’ results, where we found significant correlations between FIB and water temperature. The model was also applied to all sites separately and similar relations were found. These runs gave adjusted R 2 values between 0.19 and 0.55 for E. coli and 0.27 and 0.52 for enterococci. For most sampling sites, the variables precipitation and/or water temperature did not significantly contribute to the model.
4 Discussion
We measured and statistically analysed FIB concentrations in the Betna River of Bangladesh. The measured mean concentrations (log cfu/100 mL) of E. coli (2.9–3.4) and enterococci (3.5–4.0) are comparable to other studies conducted in developing countries, for instance, in China (E. coli 1.8–3.4) (Liu et al. 2009), India (enterococci 2.18–5.84) (Lata et al. 2016), Southeast Asia (E. coli 2.8–4.3) (Widmer et al. 2013), and Côte d’Ivoire (E. coli 2.55–3.47) (Adingra et al. 2012). In the present study, 88% of E. coli and all enterococci samples exceeded bathing water quality standards (specified by USEPA), which indicates potential health risks associated with the use of the river water for domestic, bathing and irrigation purposes. These frequent standard failures are not surprizing for the study area, as the sewers drain directly to the river without treatment. The frequent violation of water quality standards has also been reported in other studies (Noble et al. 2003; Schilling et al. 2009; Myers and Ambrose 2015). Enterococci concentrations were higher than E. coli concentrations in most of our samples, and this is also in agreement with previous studies (Shergill and Pitt 2004; Tiefenthaler et al. 2009). The observed higher levels of enterococci are explained by enterococci’s longer survival than E. coli in surface water (Liu et al. 2006). Noble et al. (2003) in their study in southern California also reported that enterococci exceeded the single sample standards most often due to enterococci’s longer survival in the marine environment compared to faecal coliform.
Significantly higher FIB concentrations were found during wet weather compared to dry weather, which is in agreement with other studies (Walters et al. 2011; Abia et al. 2015; Aragonés et al. 2016). Precipitation was positively correlated with FIB, because surface water is likely contaminated with manure through increased runoff from agricultural lands and urban areas (e.g., Satkhira town), leakage from manure storage areas and septic tanks leakage, and resuspension from sediments. Other studies (An et al. 2002; Isobe et al. 2004; Funari et al. 2012; Vermeulen and Hofstra 2013; Martinez et al. 2014; Abia et al. 2015) report similar reasons for the positive correlations between FIB concentrations and precipitation. Significantly positive correlations between FIB and precipitation indicate that diffuse sources contributed more during wet weather than during dry weather. Similarly, Ibekwe et al. (2011) also found in the Santa Ana River in southern California that storm water runoff from surrounding urban and agricultural areas is a dominant source of faecal contamination. The observed higher FIB concentrations during wet weather and intense precipitation are also consistent with findings by others (Schilling et al. 2009; Walters et al. 2011; Abia et al. 2015; Dastager 2015; Aragonés et al. 2016).
Water temperature was also positively correlated to FIB, likely because the study area is situated in a subtropical climate where June to September frequent rainfall and high summer temperatures coincide. The same positive correlation was reported in other studies (Koirala et al. 2008; Schilling et al. 2009). These authors also relate it to the coincidence of high temperature and precipitation during the summer period. Therefore, the observed positive correlation with water temperature does not mean that temperature stimulated the increased FIB level in the study area. In some other studies FIB growth was suspected to be a possible reason for the positive relation between temperature and FIB (Byappanahalli et al. 2003; Tiefenthaler et al. 2009; Hong et al. 2010; Abia et al. 2015), for instance because of decreased dissolved oxygen content, algal blooms and nutrient richness (Rouf et al. 2012). However, we have not found proof of FIB growth in the literature. In the studied river, due to the tidal in and outflowing water, the residence time of the bacteria is low. Therefore, long survival, growth and proliferation of bacteria are unlikely.
We found negative correlations between water salinity and bacterial concentrations. This is consistent with results from previous studies (Adingra et al. 2012; Hoppe et al. 2013; Dastager 2015; Aragonés et al. 2016). Water salinity depends on the amount of precipitation and associated fresh water inflow from the upstream watersheds (Hoppe et al. 2013). In our study area, during the rainy season (July to September) precipitation increases and as a result water salinity decreases. The observed negative correlation with salinity is more likely due to the typical weather pattern during the rainy season when low salinity coincides with increased precipitation and high temperature, rather than salinity dependent die-off of bacteria.
Our linear regression model explains nearly half of the variation in FIB concentration (R 2 = 0.46 for E. coli and 0.48 for enterococci) by taking climatic and environmental variables into account. The variation of R 2 depends on the climatic variables added, the number of data used, and the microbes considered (Vermeulen and Hofstra 2013). The model results compare well with other studies, for instance, Whitman and Nevers (2008) conducted a regression analysis for 23 beaches in Chicago. After adjustments for spatial and temporal autocorrelations, they found an adjusted R 2 that ranged from 0.20 to 0.41. Kay et al. (2005) reported E. coli with R 2 values of 0.49–0.68 for the river Ribble drainage basin in the UK by including similar climatic and environmental variables. Vermeulen and Hofstra (2013) reported a similar R 2 of 0.49 for E. coli in the Rhine, Meuse and Drentse Aa, and Walters et al. (2011) found a lower R 2 value of 0.15 for E. coli and 0.11 for enterococci in their regression study in central California coastal water, including similar variables compared to our study.
The presence of indicator bacteria in waterbodies does not pose a direct risk of waterborne diseases, but their presence indicates faecal contamination and the possible presence of waterborne pathogens (Burres 2009; Teklehaimanot et al. 2014). Risk of waterborne disease outbreaks also depends on water uses, such as consumption and recreational activities. The Betna River water is used for both domestic purposes (e.g., washing of clothes and utensils, and cooking) and agricultural and aquacultural production. People also come in direct contact with polluted river water during fishing and bathing. Therefore, the persistently high FIB concentrations and the intense human exposure to the contaminated river water indeed is a serious public health risk in the study area. The actual risk could be assessed by a quantitative microbial risk assessment. This requires pathogen concentration data in river water, but detection of pathogens is expensive and may cause potential health hazards (Bruhn and Wolfson 2007). Therefore, to quantify and model waterborne pathogen distribution and dynamics, and to assess associated health risk by incorporating pathogens instead of relying on indicator bacteria is challenging. FIB standards for bathing and drinking water are designed to guarantee limited disease risks. In most samples these standards are violated. This also indicates a public health concern in the study area.
We have found that FIB concentrations increase with increased temperature and precipitation. Therefore, we expect that projected increased precipitation and associated runoff under climate change will increase FIB concentrations in surface waters. We anticipate that pathogens behave similarly to FIB and as a result, health risk will increase with increase in temperature and precipitation.
5 Conclusions
Based on the analysis of a total of 96 FIB samples of water temperature, salinity and precipitation of Betna River in southwest Bangladesh, we conclude as follows:
-
Eighty-eight percent of the E. coli samples and all enterococci samples exceeded USEPA daily (single sample) bathing water quality standards. Therefore, the river Betna is unsuitable for swimming or bathing.
-
Water temperature and precipitation summed over three days correlated positively with FIB concentrations.
-
Our regression model can explain 46% of E. coli and 48% of enterococci variability in river water taking into account the variables water temperature and precipitation.
-
From our results we expect that projected increased precipitation, associated with frequent tropical cyclones, and ambient water temperature may further increase the FIB concentrations. Waterborne pathogens likely respond similarly to environmental variables. This means that disease outbreaks could also increase and even become a larger threat to public health.
References
Abia ALK, Ubomba-Jaswa E, Momba MNB (2015) Impact of seasonal variation on Escherichia coli concentrations in the riverbed sediments in the Apies River, South Africa. Sci Total Environ 537:462–469
ADB (2011) Adapting to climate change: strengthening the climate resilience of the water sector infrastructure in Khulna. Bangladesh, Asian Development Bank, Mandaluyong City, Philippines
Adingra A, Kouadio A, Blé M, Kouassi A (2012) Bacteriological analysis of surface water collected from the grand-Lahou lagoon, Côte d’Ivoire. Afr J Microbiol Res 6:3097–3105
Ahmed N, Bachofen C, Cameron E (2011) Case study-Bangladesh: climate impacts threaten national development. SDCC Learning in Focus, The World Bank, Washington DC
Ahmed W, Yusuf R, Hasan I, Goonetilleke A, Gardner T (2010) Quantitative PCR assay of sewage-associated Bacteroides markers to assess sewage pollution in an urban lake in Dhaka, Bangladesh. Can J Microbiol 56:838–845
An Y-J, Kampbell DH, Peter Breidenbach G (2002) Escherichia coli and total coliforms in water and sediments at lake marinas. Environ Pollut 120:771–778
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington DC
Aragonés L, López I, Palazón A, López-Úbeda R, García C (2016) Evaluation of the quality of coastal bathing waters in Spain through fecal bacteria Escherichia coli and enterococcus. Sci Total Environ 566:288–297
BBS (2011) Population and housing census report 2011. Bangladesh Bureau of Statistics, Ministry of Planning, Government of Bangladesh
Bruhn L, Wolfson L (2007) Bacteria and water quality. In: Lyn C (ed) Citizens Monitoring Bacteria: a Training Manual for Monitoring E. coli, 2nd edn. USA, pp 7–11
Burres E (2009) Surface water ambient monitoring program using IDEXX for fecal indicator bacteria monitoring. SWRCB-Clean Water Team, Canada
Byappanahalli MN, Shively DA, Nevers MB, Sadowsky MJ, Whitman RL (2003) Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora. FEMS Microbiol Ecol 46:203–211
CEGIS (2013) Environmental impact assessment of re-excavation of Betna River in Satkhira District for removal of drainage congestion. Bangladesh Water Development Board
Crowther J, Kay D, Wyer MD (2001) Relationships between microbial water quality and environmental conditions in coastal recreational waters: the Fylde coast, UK. Water Res 35:4029–4038
Dastager SG (2015) Assessment of enteric bacterial indicators and correlation with physico-chemical parameters in Veraval coast, India. Indian J Geo-Marine Sci 44:501–507
Delpla I, Jung A-V, Baures E, Clement M, Thomas O (2009) Impacts of climate change on surface water quality in relation to drinking water production. Environ Int 35:1225–1233
Faruque SM (2014) Food and waterborne diseases. International Centre for Diarrhea and Diarrheal Diseases of Bangladesh (ICDDR'B), Dhaka, Bangladesh. http://www.Icddrb.Org/News-and-events/features?Id=73& task=view. Accessed 12 Nov 2015
Freeman J, Anderson D, Sexton D (2009) Seasonal peaks in Escherichia coli infections: possible explanations and implications. Clin Microbiol Infect 15:951–953
Funari E, Manganelli M, Sinisi L (2012) Impact of climate change on waterborne diseases. Ann Ist Super Sanità 48:473–487
Hofstra N (2011) Quantifying the impact of climate change on enteric waterborne pathogen concentrations in surface water. Curr Opin Environ Sustain 3:471–479
Hong H, Qiu J, Liang Y (2010) Environmental factors influencing the distribution of total and faecal coliform bacteria in six water storage reservoirs in the Pearl River Delta region, China. J Environ Sci 22:663–668
Hoppe H-G, Giesenhagen H, Koppe R, Hansen H-P, Gocke K (2013) Impact of change in climate and policy from 1988 to 2007 on environmental and microbial variables at the time series station Boknis Eck, Baltic Sea. Biogeosciences 10:4529–4546
Hossain ANHA (2003) Integrated flood management: case study Bangladesh. In: WMO/GWP associated programme on flood management. Bangladesh Water Development Board, Dhaka, pp 1–14
Ibekwe AM, Lesch S, Bold R, Leddy M, Graves A (2011) Variations of indicator bacteria in a large urban watershed. T ASABE 54:2227–2236
Isobe KO, Tarao M, Chiem NH, Minh LY, Takada H (2004) Effect of environmental factors on the relationship between concentrations of coprostanol and fecal indicator bacteria in tropical (Mekong Delta) and temperate (Tokyo) freshwaters. Appl Environ Microbiol 70:814–821
IWM (2014) Feasibility study for drainage improvement of polder 1, 2, 6–8 by mathematical modelling under the Satkhira District. Institute of Water Modelling, Bangladesh
Kamal A, Goyer K, Koottatep T, Amin A (2008) Domestic wastewater management in south and Southeast Asia: the potential benefits of a decentralised approach. Urban Water J 5:345–354
Kay D et al (2005) Predicting faecal indicator fluxes using digital land use data in the UK's sentinel water framework directive catchment: the Ribble study. Water Res 39:3967–3981
Koirala SR, Gentry RW, Perfect E, Schwartz JS, Sayler GS (2008) Temporal variation and persistence of bacteria in streams. J Environ Qual 37:1559–1566
Lata P, Ram S, Shanker R (2016) Multiplex PCR based genotypic characterization of pathogenic vancomycin resistant Enterococcus faecalis recovered from an Indian river along a city landscape SpringerPlus 5:1199–1206
Liu L et al (2006) Modeling the transport and inactivation of E. coli and enterococci in the near-shore region of Lake Michigan. Environ Sci Technol 40:5022–5028
Liu Y, Zhang C, Wang X (2009) Simultaneous detection of enteric bacteria from surface waters by QPCR in comparison with conventional bacterial indicators. Environ Monit Assess 158:535–544
Lucas FS, Therial C, Gonçalves A, Servais P, Rocher V, Mouchel J-M (2014) Variation of raw wastewater microbiological quality in dry and wet weather conditions. Environ Sci Pollut Res 21:5318–5328
Martinez G, Pachepsky YA, Whelan G, Yakirevich AM, Guber A, Gish TJ (2014) Rainfall-induced faecal indicator organisms transport from manured fields: model sensitivity analysis. Environ Int 63:121–129
Montgomery D, Peck EA, Vining G (2001) Introduction to linear regression analysis. John Wiley & Sons, New York
Myers MR, Ambrose RF (2015) Salt marsh reduces fecal indicator bacteria input to coastal waters in Southern California. Bull South Calif Acad Sci 114:76–88
NIDOS (2009) Bangladesh Climate Change Factsheet. In: Network of International Development Organisations in Scotland, 1–8. Available in https://www.nidos.org.uk/news. Accessed 19 April 2014
Noble RT, Moore DF, Leecaster MK, McGee CD, Weisberg SB (2003) Comparison of total coliform, fecal coliform, and enterococcus bacterial indicator response for ocean recreational water quality testing. Water Res 37:1637–1643
Patz J, Olson S, Uejio C, HK G (2008) Disease emergence from global climate and land use change. Med Clin N Am 92:1473–1479
Rochelle-Newall E, Nguyen TMH, Le TPQ, Sengtaheuanghoung O, Ribolzi O (2015) A short review of fecal indicator bacteria in tropical aquatic ecosystems: knowledge gaps and future directions. Front Microbiol 6:1–15
Rose JB, Epstein PR, Lipp EK, Sherman BH, Bernard SM, Patz JA (2001) Climate variability and change in the United States: potential impacts on water-and foodborne diseases caused by microbiologic agents. Environ Health Perspect 109:211–220
Rouf M, Islam M, Hambrey J, Telfer T (2012) Nutrient budget of shrimp culture ponds in southwest coastal region of Bangladesh. J Aquacult Trop 27:41–51
Schilling KE, Zhang Y-K, Hill DR, Jones CS, Wolter CF (2009) Temporal variations of Escherichia coli concentrations in a large Midwestern river. J Hydrol 365:79–85
Shergill SS, Pitt R (2004) Quantification of Escherichia coli and enterococci levels in wet weather and dry weather flows. Proc Water Environ Fed 2004:746–774
Teklehaimanot GZ, Coetzee MA, Momba MN (2014) Faecal pollution loads in the wastewater effluents and receiving water bodies: a potential threat to the health of Sedibeng and Soshanguve communities, South Africa. Environ Sci Pollut Res 21:9589–9603
Tiefenthaler LL, Stein ED, Lyon GS (2009) Faecal indicator bacteria (FIB) levels during dry weather from Southern California reference streams. Environ Monit Assess 155:477–492
Tobler WR (1970) A computer movie simulating urban growth in the Detroit region. Econ Geogr 46:23–240
USEPA (2002) Method 1103.1: Escherichia coli (E. coli) in water by membrane filtration using membrane-Thermotolerant E. coli agar (mTEC). US EPA Office of water, Washington DC
USEPA (2009). Method 1106.1: enterococci in water by membrane filtration using membrane enterococcus-Esculin iron agar (mE-EIA). US EPA Office of water, Washington DC
Vermeulen L, Hofstra N (2013) Influence of climate variables on the concentration of Escherichia coli in the Rhine, Meuse, and Drentse Aa during 1985–2010. Reg Environ Chang 14:307–319
Walters SP, Thebo AL, Boehm AB (2011) Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res 45:1752–1762
Whitman RL, Nevers MB (2008) Summer E. coli patterns and responses along 23 Chicago beaches. Environ Sci tech 42:9217–9224
WHO (2008) Guidelines for drinking-water quality, 3rd edn, incorporating first and second addenda. World Health Organization, Geneva
Widmer K et al (2013) Prevalence of Escherichia coli in surface waters of Southeast Asian cities. World J Microbiol Biotechnol 29:2115–2124
Winfield MD, Groisman EA (2003) Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl Environ Microbiol 69:3687–3694
Wu J, Long S, Das D, Dorner S (2011) Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health 9:265–278
Zhang L, Seagren EA, Davis AP, Karns JS (2012) Effects of temperature on bacterial transport and destruction in bioretention media: field and laboratory evaluations. Water Environ Res 84:485–496
Acknowledgments
We thank the Ministry of Science and Technology of the Government of Bangladesh for providing a fellowship to pursue the study. We thank the Environmental Science Discipline, Khulna University, Bangladesh for providing laboratory support and the Bangladesh Water Development Board for providing precipitation data. Furthermore, we appreciate the help of Prof. R. Leemans and Dr. E.J. Bakker (Wageningen University) with data analysis and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Islam, M.M.M., Hofstra, N. & Islam, M.A. The Impact of Environmental Variables on Faecal Indicator Bacteria in the Betna River Basin, Bangladesh. Environ. Process. 4, 319–332 (2017). https://doi.org/10.1007/s40710-017-0239-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-017-0239-6