Abstract
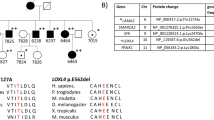
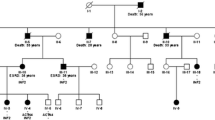
Studies on IgA nephropathy (IgAN) have identified, through GWAS, linkage analysis, and pathway scanning, molecular defects in familial and sporadic IgAN patients. In our previous study, we identified a novel variant in the SPRY2 gene that segregates with the disease in one large family. The functional characterization of this variant led us to discover that the MAPK/ERK pathway was defective not only in this family, but also in two sporadic IgAN patients wild type for SPRY2. In the present study, we have deepened the molecular analysis of the MAPK/ERK pathway and extended our evaluation to a larger cohort of sporadic patients and to one additional family. We found that the ERK pathway is defective in IgAN patients and in patients affected by another IgA-mediated disorder, Henoch-Schönlein purpura (HSP). Furthermore, we found that two other proteins, PARP1 and DNMT1, respectively involved in DNA repair and in antibody class switching and methylation maintenance duties, were critically downregulated in IgAN and HSP patients. This study opens up the possibility that defective ERK activation, in some patients, leads to PARP1 and DNMT1 downregulation suggesting that IgAN could be the consequence of a dysregulated epigenetic maintenance leading to the upregulation of several genes. In particular, PARP1 could be used as a potential biomarker for the disease.




Similar content being viewed by others
References
Donadio JV, Grande JP (2002) IgA nephropathy. N Engl J Med 347:738–748. https://doi.org/10.1056/NEJMra020109
Pozzi C (2016) Treatment of IgA nephropathy. J Nephrol 29:21–25. https://doi.org/10.1007/s40620-015-0248-3
Pozzi C, Sarcina C, Ferrario F (2016) Treatment of IgA nephropathy with renal insufficiency. J Nephrol 29:551–558. https://doi.org/10.1007/s40620-015-0257-2
Floege J (2011) The pathogenesis of IgA nephropathy: what is new and how does it change therapeutic approaches? Am J Kidney Dis 58:992–1004
Suzuki H, Kiryluk K, Novak J et al (2011) The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22:1795–1803
Hiki Y, Hori H, Yamamoto K et al (2015) Specificity of two monoclonal antibodies against a synthetic glycopeptide, an analogue to the hypo-galactosylated IgA1 hinge region. J Nephrol 28:181–186. https://doi.org/10.1007/s40620-014-0118-4
Nakagawa Y, Saito K, Nishi S et al (1999) Recurrence of IgA nephropathy 17 months after renal transplantation in the allograft transmitted thin basement membrane disease (TBMD) from donor. Clin Transpl 13(Suppl 1):59–62
Sanfilippo F, Croker BP, Bollinger RR (1982) Fate of four cadaveric donor renal allografts with mesangial IgA deposits. Transplantation 33:370–376
Iwata Y, Wada T, Uchiyama A et al (2006) Remission of IgA nephropathy after allogeneic peripheral blood stem cell transplantation followed by immunosuppression for acute lymphocytic leukemia. Intern Med 45:1291–1295
Suzuki Y, Suzuki H, Nakata J et al (2011) Pathological role of tonsillar B cells in IgA nephropathy. Clin Dev Immunol 2011:639074
Milillo A, La Carpia F, Costanzi S et al (2015) A SPRY2 mutation leading to MAPK/ERK pathway inhibition is associated with an autosomal dominant form of IgA nephropathy. Eur J Hum Genet. https://doi.org/10.1038/ejhg.2015.52
Davin JC (2011) Henoch-Schönlein purpura nephritis: pathophysiology, treatment, and future strategy. Clin J Am Soc Nephrol 6:679–689
Xu K, Zhang L, Ding J et al (2017) Value of the Oxford classification of IgA nephropathy in children with Henoch-Schönlein purpura nephritis. J Nephrol. https://doi.org/10.1007/s40620-017-0457-z
Gorelik G, Fang JY, Wu A et al (2007) Impaired T cell protein kinase C delta activation decreases ERK pathway signaling in idiopathic and hydralazine-induced lupus. J Immunol 179:5553–5563
Tordai A, Franklin RA, Patel H et al (1994) Cross-linking of surface IgM stimulates the Ras/Raf-1/MEK/MAPK cascade in human B lymphocytes. J Biol Chem 269:7538–7543
Akhiani AA, Werlenius O, Aurelius J et al (2014) Role of the ERK pathway for oxidant-induced parthanatos in human lymphocytes. PLoS One 9:e89646
Hazzalin CA, Mahadevan LC (2002) MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol 3:30–40. https://doi.org/10.1038/nrm715
Ambrose HE, Willimott S, Beswick RW et al (2009) Poly(ADP-ribose) polymerase-1 (Parp-1)-deficient mice demonstrate abnormal antibody responses. Immunology 127:178–186. https://doi.org/10.1111/j.1365-2567.2008.02921.x
Deng C, Lu Q, Zhang Z et al (2003) Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthr Rheum 48:746–756
Reale A, Matteis G, De Galleazzi G et al (2005) Modulation of DNMT1 activity by ADP-ribose polymers. Oncogene 24:13–19. https://doi.org/10.1038/sj.onc.1208005
Kauppinen TM, Chan WY, Suh SW et al (2006) Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci 103:7136–7141. https://doi.org/10.1073/pnas.0508606103
Seidl T, Whittall T, Babaahmady K, Lehner T (2012) B-cell agonists up-regulate AID and APOBEC3G deaminases, which induce IgA and IgG class antibodies and anti-viral function. Immunology 135:207–215. https://doi.org/10.1111/j.1365-2567.2011.03524.x
Schrader CE, Edelmann W, Kucherlapati R, Stavnezer J (1999) Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J Exp Med 190:323–330
Zhang Y, Zhao M, Sawalha AH et al (2013) Impaired DNA methylation and its mechanisms in CD4+ T cells of systemic lupus erythematosus. J Autoimmun 41:92–99. https://doi.org/10.1016/j.jaut.2013.01.005
Kim HS, Song M-C, Kwak IH et al (2003) Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem 278:37497–37510. https://doi.org/10.1074/jbc.M211739200
Smith SM, Tokuda S, Tsukamoto H (1992) Mucosal immune dysfunction associated with alcoholic IgA nephropathy. Clin Immunol Immunopathol 64:205–209
Acknowledgements
We would like to thank Maurizio Genuardi for critical reading of the manuscript and the rest of the Institute of Genomic Medicine for sharing discussions and ideas. We would like to thank the patients young and older for their participation to this study.
Funding
This study was funded by internal funds of the Institute of Genomic Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This project was authorized by the Comitato di Bioetica of the Catholic University (#27321/13).
Informed consent
Informed consent was obtained from all participants to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Milillo, A., Molinario, C., Costanzi, S. et al. Defective activation of the MAPK/ERK pathway, leading to PARP1 and DNMT1 dysregulation, is a common defect in IgA nephropathy and Henoch-Schönlein purpura. J Nephrol 31, 731–741 (2018). https://doi.org/10.1007/s40620-018-0482-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-018-0482-6