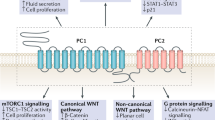
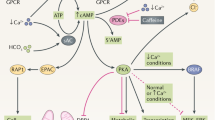
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited renal disease characterized by bilateral renal cyst formation. ADPKD is one of the most common rare disorders, accounting for ~10% of all patients with end-stage renal disease (ESRD). ADPKD is a chronic disorder in which the gradual expansion of cysts that form in a minority of nephrons eventually causes loss of renal function due to the compression and degeneration of the surrounding normal parenchyma. Numerous deranged pathways have been identified in the cyst-lining epithelia, prompting the design of potential therapies. Several of these potential treatments have proved effective in slowing down disease progression in pre-clinical animal studies, while only one has subsequently been proven to effectively slow down disease progression in patients, and it has recently been approved for therapy in Europe, Canada and Japan. Among the affected cellular functions and pathways, recent investigations have described metabolic derangement in ADPKD as a major trait offering additional opportunities for targeted therapies. In particular, increased aerobic glycolysis (the Warburg effect) has been described as a prominent feature of ADPKD kidneys and its inhibition using the glucose analogue 2-deoxy-d-glucose (2DG) proved effective in slowing down disease progression in preclinical models of the disease. At the same time, previous clinical experiences have been reported with 2DG, showing that this compound is well tolerated in humans with minimal and reversible side effects. In this work, we review the literature and speculate that 2DG could be a good candidate for a clinical trial in humans affected by ADPKD.

Similar content being viewed by others
References
Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Abad JM, Areste N, de la Torre RA, Caskey F, Couchoud C, Finne P, Heaf J, Hoitsma A, de Meester J, Pascual J, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT, Registry E-E, Euro CC, Wgikd (2014) Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 29(Suppl 4):iv15–i25. doi:10.1093/ndt/gfu017
Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, Czerwiec FS (2016) Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant. doi:10.1093/ndt/gfw240
Ong AC, Devuyst O, Knebelmann B, Walz G, Diseases E-EWGfIK (2015) Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385(9981):1993–2002. doi:10.1016/S0140-6736(15)60907-2
Grantham JJ (2008) Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359(14):1477–1485. doi:10.1056/NEJMcp0804458
Peters DJ, Sandkuijl LA (1992) Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol 97:128–139
Cornec-Le Gall E, Audrezet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Ferec C, Le Meur Y (2013) Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24(6):1006–1013. doi:10.1681/ASN.2012070650
Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363(9):820–829 pii]10.1056/NEJMoa0907419
Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363(9):830–840. doi:10.1056/NEJMoa1003491
Iliuta IA, Kitchlu A, Pei Y (2016) Methodological issues in clinical trials of polycystic kidney disease: a focused review. J Nephrol. doi:10.1007/s40620-016-0358-6
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. doi:10.1056/NEJMoa1205511
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25(34):4633–4646. doi:10.1038/sj.onc.1209597
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033. doi:10.1126/science.1160809
Menezes LF, Zhou F, Patterson AD, Piontek KB, Krausz KW, Gonzalez FJ, Germino GG (2012) Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4alpha as a disease modifier. PLoS Genet 8(11):e1003053. doi:10.1371/journal.pgen.1003053
Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19(4):488–493. doi:10.1038/nm.3092
Rowe I, Boletta A (2014) Defective metabolism in polycystic kidney disease: potential for therapy and open questions. Nephrol Dial Transplant 29(8):1480–1486. doi:10.1093/ndt/gft521
Riwanto M, Kapoor S, Rodriguez D, Edenhofer I, Segerer S, Wuthrich RP (2016) Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 11(1):e0146654. doi:10.1371/journal.pone.0146654
Hwang VJ, Kim J, Rand A, Yang C, Sturdivant S, Hammock B, Bell PD, Guay-Woodford LM, Weiss RH (2015) The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am J Physiol Renal Physiol 309 (6):F492-498. doi:10.1152/ajprenal.00238.2015
Chiaravalli M, Rowe I, Mannella V, Quilici G, Canu T, Bianchi V, Gurgone A, Antunes S, D’Adamo P, Esposito A, Musco G, Boletta A (2015) 2-Deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol. doi:10.1681/ASN.2015030231
Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X (2015) Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 125(6):2399–2412. doi:10.1172/JCI80467
Warner G, Hein KZ, Nin V, Edwards M, Chini CC, Hopp K, Harris PC, Torres VE, Chini EN (2016) Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol 27(5):1437–1447. doi:10.1681/ASN.2015020132
Goncalves S, Guerra J, Santana A, Abreu F, Mil-Homens C, Gomes da Costa A (2009) Autosomal-dominant polycystic kidney disease and kidney transplantation: experience of a single center. Transplant Proc 41(3):887–890. doi:10.1016/j.transproceed.2009.01.069
de Mattos AM, Olyaei AJ, Prather JC, Golconda MS, Barry JM, Norman DJ (2005) Autosomal-dominant polycystic kidney disease as a risk factor for diabetes mellitus following renal transplantation. Kidney Int 67(2):714–720. doi: 10.1111/j.1523-1755.2005.67132.x
Caillard S, Eprinchard L, Perrin P, Braun L, Heibel F, Moreau F, Kessler L, Moulin B (2011) Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematic screening by oral glucose tolerance test. Transplantation 91(7):757–764. doi:10.1097/TP.0b013e31820f0877
Gentil MA, Luna E, Rodriguez-Algarra G, Osuna A, Gonzalez-Molina M, Mazuecos A, Cubero JJ, Del Castillo D (2002) Incidence of diabetes mellitus requiring insulin treatment after renal transplantation in patients with hepatitis C. Nephrol Dial Transplant 17(5):887–891
Pham PT, Pham PM, Pham SV, Pham PA, Pham PC (2011) New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes 4:175–186. doi:10.2147/DMSO.S19027
Prakash J, Rathore SS, Brojen Singh T, Choudhury TA, Prabhakar, Usha (2012) New onset diabetes after transplantation (NODAT): analysis of pre-transplant risk factors in renal allograft recipients. Indian J Transplant 6(3):77–82. doi:10.1016/j.ijt.2012.07.003
Ducloux D, Motte G, Vautrin P, Bresson-Vautrin C, Rebibou JM, Chalopin JM (1999) Polycystic kidney disease as a risk factor for post-transplant diabetes mellitus. Nephrol Dial Transplant 14(5):1244–1246
Hamer RA, Chow CL, Ong AC, McKane WS (2007) Polycystic kidney disease is a risk factor for new-onset diabetes after transplantation. Transplantation 83(1):36–40. doi:10.1097/01.tp.0000248759.37146.3d
Razeghi E, Heydarian P, Amerian M, Pourmand G (2010) The risk factors for diabetes mellitus after kidney transplantation. Saudi J Kidney Dis Transpl 21(6):1038–1043
Pietrzak-Nowacka M, Safranow K, Rozanski J, Debska-Slizien A, Domanski L, Dziewanowski K, Glyda M, Jankowska M, Nocen M, Pabisiak K, Rutkowski B, Wisniewska M, Ciechanowski K (2008) Autosomal dominant polycystic kidney disease is not a risk factor for post-transplant diabetes mellitus. Matched-pair design multicenter study. Arch Med Res 39(3):312–319. doi:10.1016/j.arcmed.2007.10.003
Courivaud C, Ladriere M, Toupance O, Caillard S, Hurault de Ligny B, Ryckelynck JP, Moulin B, Rieu P, Frimat L, Chalopin JM, Chauve S, Kazory A, Ducloux D (2011) Impact of pre-transplant dialysis modality on post-transplant diabetes mellitus after kidney transplantation. Clin Transplant 25(5):794–799. doi:10.1111/j.1399-0012.2010.01367.x
Hjelmesaeth J, Hartmann A (1999) Insulin resistance in patients with adult polycystic kidney disease. Nephrol Dial Transplant 14(10):2521–2522
Ruderman I, Masterson R, Yates C, Gorelik A, Cohney SJ, Walker RG (2012) New onset diabetes after kidney transplantation in autosomal dominant polycystic kidney disease: a retrospective cohort study. Nephrology (Carlton) 17 (1):89–96. doi:10.1111/j.1440-1797.2011.01507.x
Ghisdal L, Van Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D (2012) New-onset diabetes after renal transplantation: risk assessment and management. Diabetes Care 35(1):181–188. doi:10.2337/dc11-1230
Jacquet A, Pallet N, Kessler M, Hourmant M, Garrigue V, Rostaing L, Kreis H, Legendre C, Mamzer-Bruneel MF (2011) Outcomes of renal transplantation in patients with autosomal dominant polycystic kidney disease: a nationwide longitudinal study. Transpl Int 24(6):582–587. doi:10.1111/j.1432-2277.2011.01237.x
Cheungpasitporn W, Thongprayoon C, Vijayvargiya P, Anthanont P, Erickson SB (2016) The risk for new-onset diabetes mellitus after kidney transplantation in patients with autosomal dominant polycystic kidney disease: a systematic review and meta-analysis. Can J Diabetes. doi:10.1016/j.jcjd.2016.03.001
Pietrzak-Nowacka M, Safranow K, Byra E, Nowosiad M, Marchelek-Mysliwiec M, Ciechanowski K (2010) Glucose metabolism parameters during an oral glucose tolerance test in patients with autosomal dominant polycystic kidney disease. Scand J Clin Lab Invest 70(8):561–567. doi:10.3109/00365513.2010.527012
Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M (2011) Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6(1):7–13. doi:10.2215/CJN.04140510
Vareesangthip K, Tong P, Wilkinson R, Thomas TH (1997) Insulin resistance in adult polycystic kidney disease. Kidney Int 52(2):503–508
Reed B, Helal I, McFann K, Wang W, Yan XD, Schrier RW (2012) The impact of type II diabetes mellitus in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27(7):2862–2865. doi:10.1093/ndt/gfr744
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39(2):171–183. doi:10.1016/j.molcel.2010.06.022
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20(1):51–56. doi:10.1016/j.gde.2009.10.009
Ockuly JC, Gielissen JM, Levenick CV, Zeal C, Groble K, Munsey K, Sutula TP, Stafstrom CE (2012) Behavioral, cognitive, and safety profile of 2-deoxy-2-glucose (2DG) in adult rats. Epilepsy Res 101(3):246–252. doi:10.1016/j.eplepsyres.2012.04.012
Stafstrom CE, Roopra A, Sutula TP (2008) Seizure suppression via glycolysis inhibition with 2-deoxy-d-glucose (2DG). Epilepsia 49(Suppl 8):97–100. doi:10.1111/j.1528-1167.2008.01848.x
Minor RK, Smith DL Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA (2010) Chronic ingestion of 2-deoxy-d-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol 243(3):332–339. doi:10.1016/j.taap.2009.11.025
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. doi:10.4103/0976-0105.177703
Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ (2013) A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71(2):523–530. doi:10.1007/s00280-012-2045-1
Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, Eddy S, Goodin S, White E, Dipaola RS (2010) Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 70(13):1388–1394. doi:10.1002/pros.21172
Singh D, Banerji AK, Dwarakanath BS, Tripathi RP, Gupta JP, Mathew TL, Ravindranath T, Jain V (2005) Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol 181(8):507–514. doi:10.1007/s00066-005-1320-z
Landau BR, Laszlo J, Stengle J, Burk D (1958) Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-d-glucose. J Natl Cancer Inst 21(3):485–494
Kilbourne ED (1959) Inhibition of influenza virus multiplication with a glucose antimetabolite (2-deoxy-d-glucose). Nature 183(4656):271–272
Courtney RJ, Steiner SM, Benyesh-Melnick M (1973) Effects of 2-deoxy-d-glucose on herpes simplex virus replication. Virology 52(2):447–455
Leung HJ, Duran EM, Kurtoglu M, Andreansky S, Lampidis TJ, Mesri EA (2012) Activation of the unfolded protein response by 2-deoxy-d-glucose inhibits Kaposi’s sarcoma-associated herpesvirus replication and gene expression. Antimicrob Agents Chemother 56(11):5794–5803. doi:10.1128/AAC.01126-12
Sanchez EL, Lagunoff M (2015) Viral activation of cellular metabolism. Virology 479–480:609–618. doi:10.1016/j.virol.2015.02.038
Lane MA, Mattison J, Ingram DK, Roth GS (2002) Caloric restriction and aging in primates: relevance to humans and possible CR mimetics. Microsc Res Tech 59(4):335–338. doi:10.1002/jemt.10214
Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6(4):280–293. doi:10.1016/j.cmet.2007.08.011
Che Q, Lin L, Ai Q, Ge P, Dai J, Jiang R, Zhou D, Wan J, Zhang L (2015) Caloric restriction mimetic 2-deoxyglucose alleviated lethal liver injury induced by lipopolysaccharide/d-galactosamine in mice. Biochem Biophys Res Commun 459(3):541–546. doi:10.1016/j.bbrc.2015.02.145
Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP (1999) 2-Deoxy-d-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res 57(1):48–61
Duan W, Mattson MP (1999) Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res 57(2):195–206
Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y (2014) 2-Deoxy-d-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett 355(2):176–183. doi:10.1016/j.canlet.2014.09.003
Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG (2011) Targeting glucose metabolism: an emerging concept for anticancer therapy. Am J Clin Oncol 34(6):628–635. doi:10.1097/COC.0b013e3181e84dec
Dwarakanath B, Jain V (2009) Targeting glucose metabolism with 2-deoxy-d-glucose for improving cancer therapy. Future Oncol 5(5):581–585. doi:10.2217/fon.09.44
Farooque A, Afrin F, Adhikari JS, Dwarakanath BS (2009) Protection of normal cells and tissues during radio- and chemosensitization of tumors by 2-deoxy-d-glucose. J Cancer Res Ther 5(Suppl 1):S32–S35. doi:10.4103/0973-1482.55138
Burckhardt D, Stalder GA (1975) Cardiac changes during 2-deoxy-d-glucose test. A study in patients with selective vagotomy and pyloroplasty. Digestion 12(1):1–8
Stalder GA, Schultheiss HR, Allgower M (1972) Use of 2-deoxy-d-glucose for testing completeness of vagotomy in man. Gastroenterology 63(4):552–556
Acknowledgements
We are grateful to several colleagues and friends for helpful discussions, to Angela Pesenti Gritti for careful and essential collection of the literature, to the Italian Association for Reasearch on PKD (AIRP), to all the patients and to Dr. S. Bramani for her generous and continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Boletta is co-inventor on a patent for the use of inhibitors of glycolysis including 2DG, for the treatment of Polycystic Kidney Disease.
Funding
The following agencies have provided funding: Italian Association for Polycystic Kidney Disease and their patients (AIRP, http://www.renepolicistico.it); the Italian Ministry of Health (RF2011-02351840 to AB and RF2013-02356802 to RM); the PKD Foundation (http://www.pkdcure.org) (RFA2013 187G14a/b to AB).
Ethical statement
This article does not contain any studies with human participants performed by any of the authors.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Magistroni, R., Boletta, A. Defective glycolysis and the use of 2-deoxy-d-glucose in polycystic kidney disease: from animal models to humans. J Nephrol 30, 511–519 (2017). https://doi.org/10.1007/s40620-017-0395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-017-0395-9