Abstract
Background
We recently showed that reactive oxygen species (ROS) and mitochondrial DNA damage and deletions were attenuated by postconditioning (POC). It is not known, however, whether a population of progenitor cells is recruited by POC and is responsible for repair of renal tubular epithelial cells after ischemic injury.
Methods
The model of renal POC was induced by 45 min clamping of the left renal artery and right nephrectomy followed by 7 min of short-time full reperfusion and 3 cycles of 30 s ischemia and 30 s reperfusion. The lymphocyte compartment of peripheral blood was evaluated by fluorescence-activated cell sorting (FACS) to determine expression of the bone marrow-derived progenitor cell markers CXC-chemokine receptor 4 (CXCR4), c-Kit, and CD34, at 12 h, 1 day and 3 days post-ischemia. Serum and kidney tissue were collected for analysis at 3 and 7 days post-ischemia.
Results
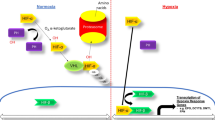
Renal functional and structural recovery was markedly improved by POC, which increased the number of CXCR4+ and CD34+ stem cells in peripheral blood and kidney tissue. Inhibition of ROS burst by POC was likely associated with increased hypoxia-inducible factor-1 expression, which may further promote stromal cell-derived factor 1 (SDF-1) expression.
Conclusions
The mechanisms of stem cell recruitment to the injured foci mobilized by POC appear to be mediated by moderate oxidative stress, which may lead to increased SDF-1 expression.





Similar content being viewed by others
References
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334:1448–1460
Gill N, Nally JV Jr, Fatica RA (2005) Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest 128:2847–2863
Maitra G, Ahmed A, Rudra A, Wankhede R, Sengupta S, Das T (2009) Renal dysfunction after off-pump coronary artery bypass surgery- risk factors and preventive strategies. Indian J Anaesth 53:401–407
Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17:1135–1142
Ali T, Khan I, Simpson W, Prescott G, Townsend J, Smith W, Macleod A (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18:1292–1298
Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17:1503–1520
Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100:157–168
Metcalf D (2001) Hematopoietic stem cells: old and new. Biomed Pharmacother 55:75–78
McCulloch EA, Till JE (2005) Perspectives on the properties of stem cells. Nat Med 11:1026–1028
Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG (2007) Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18:2486–2496
Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK (2011) Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 9:51–57
Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C (2005) Administerd mesenchymal stem cells protected against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol 289(1):F31–F42
Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS (2010) Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121:2211–2220
Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV (2005) Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow–derived stem cells. J Clin Invest 115:1743–1755
Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195:229–235
Sun Z, Zhang X, Locke JE, Zheng Q, Tachibana S, Diehl AM, Williams GM (2009) Recruitment of host progenitor cells in rat liver transplants. Hepatology 49:587–597
Liu L, Yu Q, Lin J, Lai X, Cao W, Du K, Wang Y, Wu K, Hu Y, Zhang L, Xiao H, Duan Y, Huang H (2011) Hypoxia-inducible factor-1α is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev 20(11):1961–1971
Ceradini DJ, Gurtner GC (2005) Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 15(2):57–63
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10(8):858–864
Togel FE, Westenfelder C (2011) Role of SDF-1 as a regulatory chemokine in renal regeneration after acute kidney injury. Kidney Int 1(3):87–89
Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C (2005) Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67:1772–1784
Tajima Fumihito, Sato Takashi, Laver Joseph H, Ogawa Makio (2000) CD34 expression by murine hematopoietic stem cells mobilized by granulocyte colony stimulating factor. Blood 96:1989–1993
Asahara T, Kawamoto A (2004) Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol 287:C572–C579
Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21(12):3995–4004
Pouysségur J, Mechta-Grigoriou F (2006) Redox regulation of the hypoxia inducible factor. Biol Chem 387:1337–1346
Schroedl C, McClintock DS, Budinger GR, Chandel NS (2002) Hypoxic but not anoxic stabilization of HIF1-alpha requires mitochondrial reactive oxygen species. Am J Physiol 283:L922–L931
Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG (1978) Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2 and S3 segments. Kidney Int 14:31–49
Wang W, Tang T, Zhang P, Bu H (2010) Postconditioning attenuates renal ischemia-reperfusion injury by preventing DAF down-regulation. J Urol 183:2424–2431
Tan Xiaohua, Zhang Lei, Jiang Yunpeng, Yang Yujia, Zhang Wenqi, Li Yulin, Zhang Xiuying (2013) Postconditioning attenuates mitochondrial oxidative stress in rat kidney exposed to ischemia-reperfusion injury. Nephrol Dial Transpl 28(11):2754–2765
Alfarano C, Roubeix C, Chaaya R, Ceccaldi C, Calise D, Mias C, Cussac D, Bascands JL, Parini A (2012) Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant 21(9):2009–2019
Cai J, Yu X, Xu R, Fang Y, Qian X, Liu S, Teng J, Ding X (2014) Maximum efficacy of mesenchymal stem cells in rat model of renal ischemia-reperfusion injury: renal artery administration with optimal numbers. PLoS ONE 9(3):e92347
Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG (2003) Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112:42–49
Feng G, Mao D, Che Y, Su W, Wang Y, Xu Y, Fan Y, Zhao H, Kong D, Xu Y, Li Z (2013) The phenotypic fate of bone marrow-derived stem cells in acute kidney injury. Cell Physiol Biochem 32(5):1517–1527
Segerer S, Nelson PJ, Schlondorff D (2000) Chemokines, chemokin receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11:152–176
Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S, Moore MA (2001) Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97:3354–3360
Cooper CR, Chay CH, Gendernalik JD, Lee HL, Bhatia J, Taichman RS, McCauley LK, Keller ET, Pienta KJ (2003) Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 97:739–747
Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ (2002) CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood 100:2597–2606
Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T (2002) G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and upregulating CXCR4. Nat Immunol 3:687–694
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10(8):858–864
Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR (1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393:595–599
Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC (2008) Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem 283:10930–10938
Bernhardt WM, Câmpean V, Kany S, Jürgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Günzler V, Amann K, Willam C, Wiesener MS, Eckardt KU (2006) Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17:1970–1978
Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW (2010) Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA 107:4675–4680
Koshikawa N, Hayashi J, Nakagawara A, Takenaga K (2009) Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem 284:33185–33194
Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G (2004) Ferritin heavy chain upregulation by NF-kappaB inhibits TNF-alpha-induced apoptosis by suppressing reactive oxygen species. Cell 119:529–542
Finkel T (2000) Redox-dependent signal transduction. FEBS Lett 476:52–54
Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE (2010) Hypoxia-inducible factor-1 activation in non-hypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell 21:3247–3257
Pagé EL, Robitaille GA, Pouysségur J, Richard DE (2002) Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem 277:48403–48409
Guzy RD, Schumacker PT (2006) Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91:807–819
Hsu CC, Wang CH, Wu LC, Hsia CY, Chi CW, Yin PH, Chang CJ, Sung MT, Wei YH, Lu SH, Lee HC (2013) Mitochondrial dysfunction represses HIF-1α protein synthesis through AMPK activation in human hepatoma HepG2 cells. Biochim Biophys Acta 1830(10):4743–4751
Marfella R, D’Amico M, Di Filippo C, Piegari E, Nappo F, Esposito K, Berrino L, Rossi F, Giugliano D (2002) Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia 45:1172–1181
Acknowledgments
This work was supported by National Natural Science Foundation of China Award number 81470864 and Program of New Century Excellent Talents of Ministry of Education in China, Award number NCET-10-0448.
Conflict of interest
All the authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Tan and R. Yin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tan, X., Yin, R., Chen, Y. et al. Postconditioning attenuates renal ischemia–reperfusion injury by mobilization of stem cells. J Nephrol 28, 289–298 (2015). https://doi.org/10.1007/s40620-015-0171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-015-0171-7