Abstract
Background
We describe data on 10,472 renal biopsies gathered by the Czech Registry of Renal Biopsies over a period of 18 years.
Methods
We assessed the main demographic, clinical and histological data of individuals who underwent renal biopsies of native kidneys in 31 centers in the Czech Republic (population 10.3 million) during the period 1994–2011.
Results
We evaluated 10,472 renal biopsies: males 57.8 %, children (≤15 years) 13.6 %, elderly (>60 years) 19.1 %. The most frequent biopsy-proven diseases were primary (55.7 %) and secondary (29.1 %) glomerulonephritides (GN). Tubulointerstitial nephritis (TIN) was observed in 3.4 % and vascular diseases in 4.1 %. The samples were non-diagnostic in 4.2 %. Among primary GN the most frequent diagnoses were IgA nephropathy (IgAN) (37.4 %), membranous GN (MGN) (13 %) and focal segmental glomerulosclerosis (FSGS) (12.6 %). Among secondary GN, systemic lupus erythematosus (SLE) represented 23.2 %, hereditary diseases 19.8 % and necrotizing vasculitis (NV) 19.4 %. Among adults, mild renal insufficiency [serum creatinine (SCr) 111–200 μmol/l] was present in 24.7 %, advanced renal insufficiency (SCr 201–400 μmol/l) in 15.3, and 12.3 % of patients had SCr > 400 μmol/l. The most common diseases in patients with nephrotic proteinuria were minimal change disease (MCD) (39.7 %) among children, IgAN (26.2 %) in adults aged 16–60 years and amyloidosis (42.7 %) among the elderly. The mean annual incidence (per million population) was: primary GN 30.9, secondary GN 18.1, IgAN 11.6, MGN 4.0, SLE 4.0, FSGS 3.9, MCD 3.4, NV 3.2, diabetic nephropathy 2.3, thin basement membrane glomerulopathy 2.0, mesangioproliferative GN 1.9, and TIN 1.9. Ultrasound needle guidance was used in 66.8 %. The frequency of serious complications (symptomatic hematoma, gross hematuria, blood transfusion) was approximately 3.2 %.
Conclusions
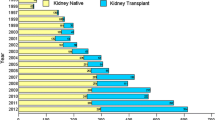
This report provides representative population-based data on native biopsy-proven renal diseases in the Czech Republic. Over the 18 years of nationwide biopsy survey, we noted an increase of the mean age of renal biopsy cases, an increasing proportion of elderly, and a cardinal change in biopsy technique towards ultrasonography needle guidance.


Similar content being viewed by others
References
Rychlík I, Jančová E, Tesař V et al (2004) The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994–2000. Nephrol Dial Transplant 19:3040–3049
Churg J, Bernstein J, Glassock R (1995) Renal disease: Classification and atlas of glomerular diseases, 2nd edn. IGAKU-SHOIN Medical Publishers, Inc. New York (ISBN: 0-89640-257-6)
Schena P, Gesualdo L (1994) Renal biopsy beyond histology and immunofluorescence. Nephrol Dial Transplant 9:1541–1544
Heaf J, Lokkegaard H, Larsen S (1999) The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 14:1889–1897
Rivera F, López-Gómez JM, Pérez-García R, Spanish registry of glomerulonephritis (2002) Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 17(9):1594–1602
Braun N, Schweisfurth A, Lohofener Ch et al (2011) Epidemiology of glomerulonephritis in Northern Germany. Int Urol Nephrol 43:1117–1126
McQuarrie EP, Mackinnon B, Young B et al (2009) Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant 24:1524–1528
Hanko JB, Mullan RN, O´Rourke DM et al (2009) The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant 24:3050–3054
Naumovic R, Pavlovic S, Stojkovic D et al (2009) Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant 24:877–885
Covic A, Schiller A, Volovat C et al (2006) Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 21:419–424
Simon P, Ramee MP, Boulahrouz R et al (2004) Epidemiologic data of primary glomerular diseases in western France. Kidney Int 66:905–908
Wirta O, Mustonen J, Helin H et al (2008) Incidence of biopsy-proven glomerulonephritis. Nephrol Dial Transplant 23:193–200
Briganti EM, Dowling J, Finlay M et al (2001) The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16:1364–1367
Okpechi I, Swanepoel Ch, Duffield M et al (2011) Patterns of renal disease in Cape Town South Africa: a 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant 26:1853–1861
Simon P, Ramée MP, Autuly V et al (1994) Epidemiology of primary glomerular disease in a French region. Variations according to period and age. Kidney Int 46:1192–1198
Coppo R, Gianoglio B, Porcellini MG et al (1998) Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Nephrol Dial Transplant 13:293–297
McGrogan A, Franssen CF, de Vries CS (2011) The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 26:414–430
Schena FP (1997) Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 12:418–426
El Reshaid W, El Reshaid K, Kapoor MM et al (2003) Glomerulopathy in Kuwait: the spectrum over the past 7 years. Ren Fail 25:619–630
Swaminathan S, Leung N, Lager DJ et al (2006) Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol 1:483–487
Fischer EG, Harris AA, Carmichael B et al (2009) IgA nephropathy in the triethnic population of New Mexico. Clin Nephrol 72:163–169
Al Arrayed A, George SM, Malik AK et al (2004) The spectrum of glomerular diseases in the kingdom of Bahrain: an epidemiological study based on renal biopsy interpretation. Transplant Proc 36:1792–1795
Al Arrayed A, Shariff S, Maamari MMA (2007) Kidney disease in Bahrain: a biopsy-based epidemiological study. Transplant Proc 4:875–878
Wyatt RJ, Julian BA, Baehler RW et al (1998) Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. Central Kentucky Region of the South-eastern United States IgA Nephropathy DATABANK Project. J Am Soc Nephrol 9:853–858
Research Group on Progressive Chronic Renal Disease (1999) Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1850 biopsied cases. Nephron 82:205–213
Vivante A, Afek A, Frenkel-Nir Y et al (2011) Persistent asymptomatic isolated microscopic haematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA 306:729
Sehic AM, Gaber LW, Roy S III et al (1997) Increased recognition of IgA nephropathy in African-American children. Pediatr Nephrol 11:435–437
Kiryluk K, Li Y, Sanna-Cherchi S et al (2012) Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8(6):e1002765. doi:10.1371/journal.pgen.1002765
Izzi C, Sanna-Cherchi S, Prati E et al (2006) Familial aggregation of primary glomerulonephritis in an Italian population isolate: Valtrompia study. Kidney Int 69:1033–1040
Kiryluk K, Novak J, Gharavi AG (2013) Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med 64:339–356
Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368(25):2402–2414
Novak J, Renfrow MB, Gharavi AG, Julian BA (2013) Pathogenesis of immunoglobulin A nephropathy. Curr Opin Nephrol Hypertens 22(3):287–294
Polito MG, deMoura RA, Kirsztajn GM (2010) An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9617 native kidney biopsies. Nephrol Dial Transplant 25:490–496
Li LS, Liu ZH (2004) Epidemiologic data of renal diseases from a single unit in China: analysis based on 13.519 renal biopsies. Kidney Int 66:920–923
Chang JH, Kim DK, Kim HW et al (2009) Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant 24:2406–2410
Huraib S, Khader AA, Shaheen FAM et al (2000) The spectrum of glomerulonephritis in Saudi Arabia: the results of the Saudi Registry. Saudi J Kidney Dis Transplant 11(3):434–441
Acknowledgments
The authors thank Jan Novak, MD, associate Prof. from University of Alabama at Birmingham, USA, for critical reading of our manuscript, and all colleagues from the participating renal units for supplying the requested data. The report was supported by the grant PRVOUK-P25/lF1/2.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Czech Registry of Renal Biopsies.
Dita Maixnerova and Eva Jancova have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Maixnerova, D., Jancova, E., Skibova, J. et al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994–2011. J Nephrol 28, 39–49 (2015). https://doi.org/10.1007/s40620-014-0090-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0090-z