Abstract
Purpose
Dysthyroid optic neuropathy (DON) is a rare sight-threatening complication of Graves’ disease. First-line treatment for DON consists of high-dose intravenous methylprednisolone (ivMP), followed by immediate orbital decompression (OD) if the response is poor or absent as recommended by the 2021 European Group on Graves’ orbitopathy guidelines. The safety and efficacy of the proposed therapy have been proven. However, consensus regarding possible therapeutic options for patients with contraindications to ivMP/OD or resistant form of disease is missing. This paper aims to provide and summarize all available data regarding possible alternative treatment strategies for DON.
Methods
A comprehensive literature search within an electronic database was performed including data published until December 2022.
Results
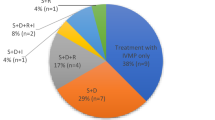
Overall, 52 articles describing use of emerging therapeutic strategies for DON were identified. Collected evidence indicates that biologics, including teprotumumab and tocilizumab, may be considered as an important possible treatment option for DON patients. Rituximab should be avoided in DON due to conflicting data and risk of adverse events. Orbital radiotherapy could be beneficial for patients with restricted ocular motility classified as poor surgical candidates.
Conclusion
Only a limited number of studies have been dedicated to the therapy of DON, mostly retrospective with a small sample size. Clear criteria regarding diagnosis and resolution of DON do not exist, which restricts comparison of therapeutic outcomes. Randomized clinical trials and comparison studies with long-term follow-ups are necessary to verify the safety and efficacy of each therapeutic option for DON.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ orbitopathy (GO) is the major extrathyroidal manifestation of Graves’ disease [1]. The pathogenesis of this autoimmune orbital disorder is based on inflammation, adipogenesis and excessive production of glycosaminoglycans, resulting in enlarged eye muscles and expansion of the orbital connective tissue [2]. It manifests with pain, diplopia, proptosis, redness and swelling of the eyelids, conjunctiva and caruncle [3, 4]. Treatment choice depends mostly on the clinical activity, severity and duration of GO.
Dysthyroid optic neuropathy (DON) is a sight-threatening complication which occurs in approximately 3–7% of patients with GO [5, 6]. Diagnosis of DON is made following clinical, ophthalmological and radiological examination. Decreased visual acuity (VA), impaired color vision, visual field defects found in clinical evaluation, as well as optic disk swelling/pallor and relative afferent pupillary defect (RAPD) revealed during ophthalmological examination are indicative of DON [7]. Magnetic resonance imaging and/or computed tomography is used to assess compression of the optic nerve exerted by swollen extraocular muscles within the orbital apex (apical crowding) and/or rarely to visualize optic nerve stretching. Differential diagnosis should exclude other orbital pathologies affecting visual functioning, such as glaucoma, cataract, orbital tumors, idiopathic orbital inflammation, orbital arteriovenous malformations, as well as rare disorders including IgG4-related ophthalmic disease and Erdheim–Chester disease [8,9,10]. However, clear criteria regarding diagnosis and resolution of DON have not been established.
First-line treatment for DON recommended by the 2021 European Group on Graves’ orbitopathy (EUGOGO) guidelines consist of high-dose intravenous methylprednisolone (ivMP) pulse therapy with 0.5–1.0 g given for 3 consecutive days or on every second day which may be repeated for another week. If the response is poor or absent, orbital decompression (OD) must be performed promptly within 1–2 weeks [11]. The described therapy is also advised in the 2022 Consensus Statement by the American Thyroid Association and European Thyroid Association [12].
So far, the majority of the published literature concerning DON provides information regarding the effectiveness of the combined approach with ivMP and decompression [13,14,15,16,17,18,19,20,21]. However, vision recovery is not always possible to achieve with the recommended therapy [16, 22, 23] and deterioration of the optic nerve function, including relapse of DON following completion of the basic treatment, has also been described [20, 24,25,26]. Moreover, the recommended therapy cannot be applied in all patients or must be discontinued due to various contraindications and side effects [17, 27,28,29,30,31], especially in those with preexisting comorbidities [32, 33].
Clinical deterioration of ophthalmic signs and symptoms in moderate-to-severe and active GO requires implementation of second-line treatment. But, when it comes to DON, alternative treatment strategies are missing and only a limited number of studies have been dedicated to the therapy of DON, mostly retrospective with a small sample size. Therefore, as optimal therapy must be applied immediately due to possible permanent dysfunction in the optic nerve caused by any delay, managing patients with DON still remains a major challenge.
In this paper, we present and review emerging alternative treatments for DON.
Dysthyroid optic neuropathy: basic treatment
Intravenous methylprednisolone and orbital decompression
First-line treatment for DON comprises of high-dose ivMP (0.5–1.0 g) pulses given for 3 consecutive days or on every second day, repeated if necessary, and followed by immediate OD in case of insufficient response or disease deterioration. Several studies have analyzed the effectiveness of ivMP therapy in various doses combined with OD [15, 17,18,19,20], but only four studies, including one prospective, have described the efficacy of the recommended DON therapy [13, 14, 16, 22]. As clear criteria of DON resolution have not been established, diverse indicators of DON improvement have been applied in the published literature, which restricts comparison of the therapeutic outcomes. However, as DON may lead to irreversible loss of vision, crucial treatment decisions depend mostly on improvement in VA.
Studies evaluating the recommended protocol described response (defined as VA 0.0–0.3 logMAR) rate to high-dose ivMP between 22 and 61%. Following OD, positive response increased to 67–87%. Additionally, randomized clinical trial (RCT) by Wakelkamp et al. [13] found that ivMP is a preferable treatment choice over OD. Surgery performed primarily did not result in a better outcome, and 83% patients required further ivMP pulses. In contrast, only 44% of patients treated first with ivMP needed subsequent OD.
Surgical decompression is conducted to directly reduce pressure within the orbital apex by extending the available orbital volume through orbital wall removal, which may be combined with fat excision in case of its hypertrophy. Inferomedial wall removal is generally considered the first approach in DON patients, as it reaches deeper into the apical complex where compression of the optic nerve is produced [34]. Several surgical approaches have been described, including transnasal, transcaruncular, transcutaneous and transantral. Recent studies show that transnasal endoscopic (TEOD) approach has become a widely used and efficient method when decompressing medial or inferior wall in patients with DON. It provides good visualization and access to the orbital apex, causing less damage to the muscles and ligaments without producing external scar [35,36,37,38,39,40,41]. Additionally, TEOD was described to result in significantly greater improvement in VA in DON patients compared to other popular approach, such as transcaruncular [42,43,44]. Severe cases may require additional lateral decompression which results in greater proptosis reduction [45,46,47,48].
According to Wakelkamp et al. [13], OD must be applied immediately in patients refractory to ivMP or with deterioration of clinical condition. However, direct indications regarding optimal timing for performing OD are missing. The World Health Organization defines mild vision impairment as VA > 0.3 logMAR, and moderate as VA > 0.5 logMAR. Additional factors such as older age, smoking, long-lasting disease, history of resistance to ivMP, poor initial VA, optic disk swelling, unstable thyroid function and high level of thyrotropin receptor antibodies have been described to be associated with potential need for immediate OD [14, 49]. Therefore, as rapid treatment decisions and personalized approach are required, patients should be referred to highly specialized centers with large surgical experience where combined endocrine, ophthalmological and surgical management is available.
Dysthyroid optic neuropathy: alternative treatments
Diverse criteria of DON resolution were applied in the analyzed literature. Successful therapy was defined as disease stabilization with no need of further DON treatment with improvement in VA and/or color vision/visual field/RAPD.
Additional intravenous methylprednisolone pulses
According to the 2021 EUGOGO guidelines, cumulative dose of ivMP in DON therapy should not exceed 8.0 g per cycle due to increased rate of adverse events [11]. However, the end point of the basic therapy with high-dose ivMP and OD has not been determined due to lack of specific criteria of DON resolution, and consensus regarding the optimal moment for performing OD is also missing. Signs of DON may persist even following the recommended therapy (clinical: decreased VA, reduced color vision, visual field defects; ophthalmological: swollen or pale optic disk, RAPD; radiological: apical crowding, optic nerve stretching).
Several studies have described the positive results obtained by applying additional ivMP pulses, both in patients with partial response to the first-line treatment [14, 16, 19] and in cases of persistent DON despite medical and surgical decompression [22]. A recently published study [50] found that applying additional ivMP pulses in a 12-week protocol (4.5 g or 7.5 g), once the DON treatment with high-dose ivMP with/without OD is completed, provides stabilization or further improvement of clinical outcome, including VA, color vision, clinical activity score (CAS) and proptosis. Further ivMP pulses may also impact quality of life of DON patients [51].
Neither of the reports described significant adverse events, although cumulative doses of ivMP exceeding 8.0 g were applied. However, as various studies proved ivMP to be associated with multiple side effects, thorough examination of patients, including assessment of aspartate and alanine aminotransferase serum levels, and blood pressure measurements, must be performed prior to administration of each pulse.
Teprotumumab
The insulin-like growth factor 1 receptor (IGF-1R) plays a critical role in the expansion of the orbital tissues in patients with GO due to its overexpression in orbital fibroblasts and immune cells [52,53,54]. Teprotumumab, a human monoclonal antibody directed against IGF-1R, is the first FDA-approved treatment for active GO, administered intravenously every 3 weeks in eight infusions with an initial dose of 10 mg/kg followed by 20 mg/kg [55]. The 2021 EUGOGO guidelines suggest applying teprotumumab as second-line treatment for moderate-to-severe and active GO.
Although patients with DON were excluded from the phase 2 and 3 clinical trials [53, 55], studies demonstrated reduction in the extraocular muscle size, also within the orbital apex, as well as significant improvement in CAS, proptosis, diplopia and quality of life in patients with GO compared to placebo, making teprotumumab a potential treatment option for DON.
To date, eight reports with a total number of 20 DON patients (29 eyes) managed with teprotumumab have been published [56,57,58,59,60,61,62,63] (Tables 1 and 2).
Teprotumumab was successful in 17 individuals (23 eyes). In 16 of them (22 eyes), teprotumumab was used as an alternative treatment. Previous therapy with ivMP with or without OD/orbital radiotherapy (ORT) failed or had to be discontinued due to intolerance. Three patients (6 eyes) resistant to teprotumumab had long-lasting DON (over 12 months) and were previously treated with ivMP, two of them also with OD.
The largest case series mentioned above [63] included 10 patients with DON (17 eyes) resistant to previous therapy. Teprotumumab (8 infusions; 10 mg/kg first infusion; 20 mg/kg for subsequent infusions) applied as alternative treatment resulted in significant improvement in VA, CAS and proptosis. Most patients experienced early response within two infusions. This rapid improvement is consistent with the clinical trials [53, 55].
Tocilizumab
Interleukin-6 (IL-6) is a pro-inflammatory cytokine found in higher concentrations in patients with GO. Through T and B cell activation, IL-6 induces adipogenesis and synthesis of glycosaminoglycans promoting expansion of the orbital volume [64,65,66]. Tocilizumab (TCZ) is a recombinant humanized monoclonal antibody directed against the IL-6 receptor, which, according to the 2021 EUGOGO guidelines, may be considered as second-line treatment for moderate-to-severe and active GO [11].
TCZ (8 mg/kg in 4 monthly infusions) was found in RCT [67] to significantly reduce CAS and median proptosis values in patients with moderate-to-severe and active GO refractory to ivMP compared to placebo. Although generally well tolerated, a higher rate of infections and headaches was noted in patients treated with TCZ. Improvement in CAS, proptosis and diplopia following TCZ infusions was also described in a few retrospective studies [68,69,70]. One of the papers noted significant improvement in VA following first month of TCZ treatment [70]. These findings highlight the potential utility of TCZ in DON patients.
Until now, seven reports with a total number of 14 DON patients (16 eyes) treated with TCZ have been published (Tables 3 and 4) [70,71,72,73,74,75,76].
TCZ (4–8 infusions) was successful in 6 patients (8 eyes). In five of them (6 eyes), TCZ was applied as alternative treatment following unsuccessful therapy with ivMP with or without OD/ORT [71,72,73,74,75]. One patient (2 eyes) received TCZ as first-line treatment with no previous use of glucocorticoids (GCs) [71].
Alternative treatment with TCZ was insufficient in 8 patients (8 eyes) resistant to ivMP and OD. A group of seven of them (7 eyes) was analyzed together in one retrospective study which found no significant improvement in VA after 1 year of treatment with TCZ [70, 76].
Rituximab
Rituximab (RTX) is a chimeric mouse–human monoclonal antibody directed against the CD20 antigen on B lymphocytes resulting in depletion of circulating B lymphocytes and transient (4–6 months) immunosuppression [77]. The 2021 EUGOGO guidelines [11] suggest considering RTX as second-line treatment for moderate-to-severe and active GO excluding patients with potential risk of developing DON. Although current recommendations do not address the use of RTX in DON patients, previous guidelines clearly advised against its administration due to conflicting data and risk of adverse events [78].
To date, four reports (overall 43 patients) described the occurrence of DON in 8 patients (18.6%; number of eyes missing) with moderate-to-severe and active GO following RTX infusions in various doses [79,80,81,82]. Additionally, two studies (overall 32 patients) evaluating the efficacy of RTX in moderate-to-severe and active GO observed 3 cases (9.1%) of severe cytokine release syndrome causing vision impairment with marked periorbital edema [81, 83].
Nevertheless, due to promising results in moderate-to-severe and active GO [83, 84] and despite potential adverse events, six reports describing 13 DON patients (number of eyes missing) treated with RTX have been published [83, 85,86,87,88,89].
In 11 DON cases, alternative combination treatment with RTX (various doses: 0.01–2.0 g) and GCs/ORT/OD was successful. Previous therapy with ivMP with/without OD failed [83, 87,88,89]. RTX (2 × 1.0 g) was insufficient in 2 cases, either as first-line treatment for DON or following ivMP pulses (3.0 g). Although transient (2–4 months) improvement in VA was achieved following RTX, further OD was required due to recurrence of DON with deterioration of vision [85, 86].
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an inosine monophosphate dehydrogenase inhibitor, which suppresses proliferation of B and T lymphocytes, decreasing the production of antibodies. MMF induces apoptosis of activated T lymphocytes, reducing chemotaxis and immune cell recruitment [90]. Moreover, it disturbs fibroblast function and proliferation [91, 92]. The 2021 EUGOGO guidelines [11] decided to include MMF into the first-line treatment for moderate-to-severe and active GO in combination with ivMP, as it was found to result in significantly greater improvement in CAS and orbital signs and symptoms compared to ivMP alone [93, 94]. This highlights the potential utility of including MMF into the basic DON treatment, but consensus regarding the use of MMF in DON is missing.
However, two reports (overall 136 patients) [94, 95] evaluating the safety and efficacy of MMF (0.72 g daily for 24 weeks) in combination with ivMP pulses (1.5–4.5 g) in patients with moderate-to-severe and active GO observed occurrence of DON in 12 patients (8.8%; number of eyes missing) during the treatment.
So far, only one study [96] evaluated the effects of additional treatment with MMF in ten DON patients previously treated with ivMP with or without OD/ORT (various doses). MMF was applied during or directly after ivMP for a median time of 76 weeks. Significant improvement was observed only in CAS. Overall, three patients experienced relapse of DON within 78 weeks from baseline.
Orbital radiotherapy
Ionizing radiation induces cell death by breaking double-stranded DNA [97]. Radiotherapy may interfere with the natural course of GO through apoptosis and disruption in functioning of orbital fibroblasts, macrophages and lymphocytes, which play a crucial role in the pathophysiology of the disease [2]. The 2021 EUGOGO guidelines recommend a combination of ORT with GCs as second-line treatment for moderate-to-severe and active GO, especially in patients with reduced eye muscle motility, excluding those with diabetic or hypertensive retinopathy and younger than 35 years [11]. According to ATA, including ORT into DON treatment may be considered [12]. Nevertheless, clear consensus regarding the use of ORT in DON patients is missing.
Several retrospective studies described the use of radiotherapy in DON patients, but they are mostly limited to a small sample size with distinct concurrent treatments, usually with oral GCs and OD [98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. So far, only one study described the use of ORT in combination with ivMP (20 Gy and 4.5 g) in a total number of 9 DON patients (number of eyes missing) obtaining resolution of DON in all cases with no recurrence during the 12-month follow-up and no major side effects [113].
The largest case series (104 patients, 163 eyes) described resolution of DON, defined as no need of urgent OD during acute phase of the disease in 98 patients (94.2%) and 152 eyes (93.3%) treated with oral GCs and ORT (20 Gy) as first-line treatment. Significant improvement in VA, color vision, visual field and RAPD was observed. Nevertheless, the retrospective character of the study with a long period of patient recruitment (22 years) should be considered while evaluating the results [98].
Moreover, ORT in addition to ivMP (20 Gy and 4.5 g) was described by Shams et al. [114] to prevent occurrence of DON in moderate-to-severe and active GO. None of the 91 patients developed DON, following combined therapy compared to 25 (17%) patients receiving ivMP alone (after average time of 3.2 years).
In contrast, ORT may also cause transient swelling of the orbital connective tissue, which may result in deterioration of DON despite simultaneous administration of ivMP (6 Gy and 3 g) as described by Hersh et al. [108].
Limitations of the study
-
1.
Clear criteria of DON recognition and resolution do not exist, which restricts comparison of the therapeutic outcomes.
-
2.
Data evaluating management of DON is scarce and comprised mostly of retrospective studies, small case series, case reports and only a few RCTs.
-
3.
Analyzed reports apply various criteria of DON resolution and provide limited information regarding follow-up results.
Conclusions
-
1.
High-dose ivMP, followed by OD remains the treatment of choice in DON as described in the 2021 EUGOGO guidelines.
-
2.
Biologics, including FDA-approved teprotumumab and TCZ, may be considered as an important treatment option for DON.
-
3.
ORT can be considered in DON patients classified as poor surgical candidates with restricted ocular motility.
-
4.
RTX should be avoided in patients with DON due to conflicting data and risk of adverse events.
-
5.
Consensus regarding alternative treatment strategies for DON is missing and its establishment is highly required. Further RCTs and comparison studies with long-term follow-ups are necessary to evaluate the safety and effectiveness of each therapeutic option in the treatment of DON.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML (2020) Epidemiology, natural history, risk factors, and prevention of graves’ orbitopathy. Front Endocrinol 11:615993
Wiersinga WM (2011) Autoimmunity in graves’ ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab 96(8):2386–2394
Neag EJ, Smith TJ (2022) 2021 update on thyroid-associated ophthalmopathy. J Endocrinol Invest 45(2):235–259
Kahaly GJ (2020) Management of graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab 105(12):3704–3720
Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA et al (1996) Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 121(3):284–290
Blandford AD, Zhang D, Chundury RV, Perry JD (2017) Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 12(2):111–121
Dolman PJ (2021) Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest 44(3):421–429
Pelewicz-Sowa M, Kajfasz M, Maślińska M, Szczepański M, Pelewicz K, Miśkiewicz P (2022) IgG4-related disease: sight-threatening orbital disease, spectacular improvement after Rituximab therapy. Pol Arch Intern Med. 133(1):16360
Chrostowska P, Drozd-Sokołowska J, Miśkiewicz P (2022) Erdheim-Chester disease with orbital involvement and progressive impairment of vision. Polish Arch Intern Med. 132(4):16193
Marinò M, Ionni I, Lanzolla G, Sframeli A, Latrofa F, Rocchi R et al (2020) Orbital diseases mimicking Graves’ orbitopathy: a long-standing challenge in differential diagnosis. J Endocrinol Invest 43(4):401–411
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C et al (2021) The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol 185(4):G43-67
Burch HB, Perros P, Bednarczuk T, Cooper DS, Dolman PJ, Leung AM et al (2022) Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Eur Thyroid J. https://doi.org/10.1530/ETJ-22-0189
Wakelkamp IMMJ, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM (2005) Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves’ ophthalmopathy? A randomized controlled trial. Clin Endocrinol 63(3):323–328
Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S et al (2014) Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid 24(5):897–905
Xu J, Ye H, Chen G, Chen J, Chen R, Yang H (2020) The therapeutic effect of combination of orbital decompression surgery and methylprednisolone pulse therapy on patients with bilateral dysthyroid optic neuropathy. J Ophthalmol 2020:9323450
Wen Y, Yan J-H (2019) The effect of intravenous high-dose glucocorticoids and orbital decompression surgery on sight-threatening thyroid-associated ophthalmopathy. Int J Ophthalmol 12(11):1737–1745
Rezar-Dreindl S, Papp A, Baumann A, Neumayer T, Eibenberger K, Stifter E et al (2022) Management of patients with dysthyroid optic neuropathy treated with intravenous corticosteroids and/or orbital decompression surgery. Graefe’s Arch Clin Exp Ophthalmol. 260(11):3683–3691
Garip Kuebler A, Wiecha C, Reznicek L, Klingenstein A, Halfter K, Priglinger S et al (2020) Evaluation of medical and surgical decompression in patients with dysthyroid optic neuropathy. Eye 34(9):1702–1709
Tramunt B, Imbert P, Grunenwald S, Boutault F, Caron P (2019) Sight-threatening Graves’ orbitopathy: Twenty years’ experience of a multidisciplinary thyroid-eye outpatient clinic. Clin Endocrinol 90(1):208–213
Jeon C, Shin JH, Woo KI, Kim Y-D (2012) Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol 26(2):73–79
Guy JR, Fagien S, Donovan JP, Rubin ML (1989) Methylprednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology 96(7):1043–1048
Miśkiewicz P, Rutkowska B, Jabłońska A, Krzeski A, Trautsolt-Jeziorska K, Kęcik D et al (2016) Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynol Pol 67(2):166–173
Zhang-Nunes SX, Dang S, Garneau HC, Hwang C, Isaacs D, Chang S-H et al (2015) Characterization and outcomes of repeat orbital decompression for thyroid-associated orbitopathy. Orbit 34(2):57–65
Ph Mourits M, Kalmann R, Sasim IV (2001) Methylprednisolone pulse therapy for patients with dysthyroid optic neuropathy. Orbit 20(4):275–280
Kauh CY, Gupta S, Douglas RS, Elner VM, Nelson CC, Niziol LM et al (2015) Compressive optic neuropathy and repeat orbital decompression: a case series. Ophthal Plast Reconstr Surg 31(5):385–390
Wenz R, Levine MR, Putterman A, Bersani T, Feldman K (1994) Extraocular muscle enlargement after orbital decompression for Graves’ ophthalmopathy. Ophthal Plast Reconstr Surg 10(1):34–41
Miśkiewicz P, Kryczka A, Ambroziak U, Rutkowska B, Główczyńska R, Opolski G et al (2014) Is high dose intravenous methylprednisolone pulse therapy in patients with Graves’ orbitopathy safe? Endokrynol Pol 65(5):402–413
Zang S, Ponto KA, Kahaly GJ (2011) Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96(2):320–332
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J et al (2012) Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol 166(2):247–253
Garrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, DeSanto LW et al (1993) Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol 116(5):533–547
Jernfors M, Välimäki MJ, Setälä K, Malmberg H, Laitinen K, Pitkäranta A (2007) Efficacy and safety of orbital decompression in treatment of thyroid-associated ophthalmopathy: long-term follow-up of 78 patients. Clin Endocrinol (Oxf) 67(1):101–107
Marinó M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14(5):403–406
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17(4):357–362
Jefferis JM, Jones RK, Currie ZI, Tan JH, Salvi SM (2018) Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye 32(3):626–636
Zah-Bi G, Abeillon-du Payrat J, Vie AL, Bournaud-Salinas C, Jouanneau E, Berhouma M (2019) Minimal-access endoscopic endonasal management of dysthyroid optic neuropathy: the dysthone study. Neurosurgery 85(6):E1059–E1067
Poślednik KB, Czerwaty K, Ludwig N, Molińska-Glura M, Jabłońska-Pawlak A, Miśkiewicz P et al (2022) Treatment results of endoscopic transnasal orbital decompression for graves’ orbitopathy-a single-center retrospective analysis in 28 orbits of 16 patients. J Pers Med. 12(10):1714
Tu Y, Xu M, Kim AD, Wang MTM, Pan Z, Wu W (2021) Modified endoscopic transnasal orbital apex decompression in dysthyroid optic neuropathy. Eye Vis 8(1):19
Singh S, Curragh DS, Selva D (2019) Augmented endoscopic orbital apex decompression in dysthyroid optic neuropathy. Eye 33(10):1613–1618
Lv Z, Selva D, Yan W, Daniel P, Tu Y, Wu W (2016) Endoscopical orbital fat decompression with medial orbital wall decompression for dysthyroid optic neuropathy. Curr Eye Res 41(2):150–158
Schaefer SD, Soliemanzadeh P, Della Rocca DA, Yoo G-P, Maher EA, Milite JP et al (2003) Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope 113(3):508–513
Kingdom TT, Davies BW, Durairaj VD (2015) Orbital decompression for the management of thyroid eye disease: an analysis of outcomes and complications. Laryngoscope 125(9):2034–2040
Nishimura K, Takahashi Y, Katahira N, Uchida Y, Ueda H, Ogawa T (2019) Visual changes after transnasal endoscopic versus transcaruncular medial orbital wall decompression for dysthyroid optic neuropathy. Auris Nasus Larynx 46(6):876–881
Shorr N, Baylis HI, Goldberg RA, Perry JD (2000) Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology 107(8):1459–1463
Perry JD (2006) Transcaruncular orbital decompression: an alternate procedure for graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol 142:889 (Author reply 889-90)
Korkmaz S, Konuk O (2016) Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res 41(2):159–164
Cheng S-N, Yu Y-Q, You Y-Y, Chen J, Pi X-H, Wang X-H et al (2021) Comparison of 2-wall versus 3-wall orbital decompression against dysthyroid optic neuropathy in visual function: a retrospective study in a Chinese population. Medicine 100(8):e24513
Dallan I, Cristofani-Mencacci L, Fiacchini G, Benettini G, Picariello M, Lanzolla G et al (2022) Functional outcomes and complications in refractory dysthyroid optic neuropathy management: Experience with 3 different surgical protocols. Am J Otolaryngol 43(3):103451
Choe CH, Cho RI, Elner VM (2011) Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthal Plast Reconstr Surg 27(1):4–11
Tagami M, Honda S, Azumi A (2020) Preoperative clinical factors and visual outcomes following orbital decompression with dysthyroid optic neuropathy. BMC Ophthalmol 20(1):30
Pelewicz M, Rymuza J, Pelewicz K, Miśkiewicz P (2022) Dysthyroid optic neuropathy: treatment with additional intravenous methylprednisolone pulses after the basic schedule is associated with stabilization or further improvement of clinical outcome. J Clin Med 11(8):2068
Pelewicz M, Rymuza J, Pelewicz K, Miśkiewicz P (2021) Impact of additional intravenous methylprednisolone pulse therapy on the quality of life in patients with dysthyroid optic neuropathy. J Med Sci [Internet]. 28;90(2 SE-Original Papers):e519. Available from: https://jms.ump.edu.pl/index.php/JMS/article/view/519. Accessed 28 Nov 2022
Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A et al (2008) Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol 181(6):4397–4405
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA et al (2017) Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 376(18):1748–1761
Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D et al (2008) B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol 181(8):5768–5774
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R et al (2020) Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 382(4):341–352
Hwang CJ, Nichols EE, Chon BH, Perry JD (2022) Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. Eur J Ophthalmol 32(3):NP46–NP49
Cheng OT, Schlachter DM (2022) Teprotumumab in advanced reactivated thyroid eye disease. Am J Ophthalmol Case Rep. 26:101484
Sears CM, Azad AD, Dosiou C, Kossler AL (2021) Teprotumumab for dysthyroid optic neuropathy: early response to therapy. Ophthal Plast Reconstr Surg 37(3S):S157–S160
Lopez MJ, Herring JL, Thomas C, Bertram BA, Thomas DA (2022) Visual recovery of dysthyroid optic neuropathy with teprotumumab. J Neuro-ophthalmol 42(2):e491–e493
Slentz DH, Smith TJ, Kim DS, Joseph SS (2021) Teprotumumab for optic neuropathy in thyroid eye disease. JAMA Ophthalmol. 139:244–247
Chiou CA, Reshef ER, Freitag SK (2021) Teprotumumab for the treatment of mild compressive optic neuropathy in thyroid eye disease: a report of two cases. Am J Ophthalmol Case Rep. 22:101075
Diniz SB, Cohen LM, Roelofs KA, Rootman DB (2021) Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthal Plast Reconstr Surg 37(6):583–591
Sears CM, Wang Y, Bailey LA, Turbin R, Subramanian PS, Douglas R et al (2021) Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: a multicenter study. Am J Ophthalmol Case Rep. 23:101111
Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y (2000) Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 85(3):1194–1199
Slowik M, Urbaniak-Kujda D, Bohdanowicz-Pawlak A, Kapelko-Slowik K, Dybko J, Wolowiec D et al (2012) CD8+CD28-lymphocytes in peripheral blood and serum concentrations of soluble interleukin 6 receptor are increased in patients with Graves’ orbitopathy and correlate with disease activity. Endocr Res 37(2):89–95
Lehmann GM, Feldon SE, Smith TJ, Phipps RP (2008) Immune mechanisms in thyroid eye disease. Thyroid 18(9):959–965
Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM et al (2018) Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 195:181–190
Pérez-Moreiras JV, Varela-Agra M, Prada-Sánchez MC, Prada-Ramallal G (2021) Steroid-resistant graves’ orbitopathy treated with tocilizumab in real-world clinical practice: a 9-year single-center experience. J Clin Med 10(4):706
Ceballos-Macías José J, Rivera-Moscoso R, Flores-Real Jorge A, Vargas-Sánchez J, Ortega-Gutiérrez G, Madriz-Prado R et al (2020) Tocilizumab in glucocorticoid-resistant graves orbitopathy. A case series report of a mexican population. Ann Endocrinol 81(2–3):78–82
Sánchez-Bilbao L, Martínez-López D, Revenga M, López-Vázquez Á, Valls-Pascual E, Atienza-Mateo B et al (2020) Anti-IL-6 receptor tocilizumab in refractory graves’ orbitopathy: national multicenter observational study of 48 patients. J Clin Med 9(9):2816
Pascual-Camps I, Molina-Pallete R, Bort-Martí MA, Todolí J, España-Gregori E (2018) Tocilizumab as first treatment option in optic neuropathy secondary to Graves’ orbitopathy. Orbit 37(6):450–453
Mehmet A, Panagiotopoulou EK, Konstantinidis A, Papagoras C, Skendros P, Dardabounis D et al (2021) Α case of severe thyroid eye disease treated with tocilizumab. Acta Medica Hradec Kral. 64(1):64–69
Sy A, Eliasieh K, Silkiss RZ (2017) Clinical response to tocilizumab in severe thyroid eye disease. Ophthal Plast Reconstr Surg 33(3):e55–e57
Gómez Rodríguez L, Cárdenas Aranzana MJ, Avilés MC (2014) Effectiveness and safety of tocilizumab in corticoid refractory Graves’ orbitopathy. Farm Hosp 38:448–450
Maldiney T, Deschasse C, Bielefeld P (2020) Tocilizumab for the management of corticosteroid-resistant mild to severe Graves’ ophthalmopathy, a report of three cases. Ocul Immunol Inflamm 28:281–284
Kaplan D, Erickson B, Kossler A, Chen J, Dosiou C (2020) SAT-500 response to tocilizumab retreatment in refractory thyroid eye disease. J Endocr Soc. 4(1):SAT-500
Salvi M, Vannucchi G, Beck-Peccoz P (2013) Potential utility of rituximab for Graves’ orbitopathy. J Clin Endocrinol Metab 98(11):4291–4299
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C et al (2016) The 2016 European thyroid association/European group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 5(1):9–26
Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 100(2):432–441
Krassas GE, Stafilidou A, Boboridis KG (2010) Failure of rituximab treatment in a case of severe thyroid ophthalmopathy unresponsive to steroids. Clin Endocrinol 72:853–855
Vannucchi G, Campi I, Covelli D, Currò N, Lazzaroni E, Palomba A et al (2021) efficacy profile and safety of very low-dose rituximab in patients with Graves’ orbitopathy. Thyroid 31(5):821–828
Insull EA, Sipkova Z, David J, Turner HE, Norris JH (2019) Early low-dose rituximab for active thyroid eye disease: an effective and well-tolerated treatment. Clin Endocrinol 91(1):179–186
Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100(2):422–431
Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S et al (2007) Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol 156(1):33–40
Salvi M, Vannucchi G, Campi I, Currò N, Simonetta S, Covelli D et al (2009) Rituximab treatment in a patient with severe thyroid-associated ophthalmopathy: effects on orbital lymphocytic infiltrates. Clin Immunol 131(2):360–365
Gess AJ, Silkiss RZ (2014) Orbital B-lymphocyte depletion in a treatment failure of rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg 30(1):e11–e13
Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ et al (2013) The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves’ orbitopathy. Clin Endocrinol 79(3):437–442
Khanna D, Chong KKL, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC et al (2010) Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology 117(1):133-139.e2
Zhang B, Li Y, Xu W, Peng B, Yuan G (2020) Use of rituximab after orbital decompression surgery in two Grave’s ophthalmopathy patients progressing to optic neuropathy. Front Endocrinol 11:583565
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118
Roos N, Poulalhon N, Farge D, Madelaine I, Mauviel A, Verrecchia F (2007) In vitro evidence for a direct antifibrotic role of the immunosuppressive drug mycophenolate mofetil. J Pharmacol Exp Ther 321(2):583–589
Azzola A, Havryk A, Chhajed P, Hostettler K, Black J, Johnson P et al (2004) Everolimus and mycophenolate mofetil are potent inhibitors of fibroblast proliferation after lung transplantation. Transplantation 77(2):275–280
Ye X, Bo X, Hu X, Cui H, Lu B, Shao J et al (2017) Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol 86(2):247–255
Kahaly GJ, Riedl M, König J, Pitz S, Ponto K, Diana T et al (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6(4):287–298
Riedl M, Kuhn A, Krämer I, Kolbe E, Kahaly GJ (2016) Prospective, systematically recorded mycophenolate safety data in Graves’ orbitopathy. J Endocrinol Invest 39(6):687–694
Quah Qin Xian N, Alnahrawy A, Akshikar R, Lee V (2021) Real-world efficacy and safety of mycophenolate mofetil in active moderate-to-sight-threatening thyroid eye disease. Clin Ophthalmol 15:1921–1932
Grudzenski S, Raths A, Conrad S, Rübe CE, Löbrich M (2010) Inducible response required for repair of low-dose radiation damage in human fibroblasts. Proc Natl Acad Sci U S A 107(32):14205–14210
Gold KG, Scofield S, Isaacson SR, Stewart MW, Kazim M (2018) Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthal Plast Reconstr Surg 34(2):172–177
Kazim M, Trokel S, Moore S (1991) Treatment of acute Graves orbitopathy. Ophthalmology 98(9):1443–1448
Li Yim JFT, Sandinha T, Kerr JM, Ritchie D, Kemp EG (2011) Low dose orbital radiotherapy for thyroid eye disease. Orbit 30(6):269–274
Beckendorf V, Maalouf T, George JL, Bey P, Leclere J, Luporsi E (1999) Place of radiotherapy in the treatment of Graves’ orbitopathy. Int J Radiat Oncol Biol Phys 43(4):805–815
Ravin JG, Sisson JC, Knapp WT (1975) Orbital radiation for the ocular changes of Gravess’ disease. Am J Ophthalmol 79(2):285–288
Covington EE, Lobes L, Sudarsanam A (1977) Radiation therapy for exophthalmos: report of seven cases. Radiology 122(3):797–799
Panzo GJ, Tomsak RL (1983) A retrospective review of 26 cases of dysthyroid optic neuropathy. Am J Ophthalmol 96(2):190–194
Claridge KG, Ghabrial R, Davis G, Tomlinson M, Goodman S, Harrad RA et al (1997) Combined radiotherapy and medical immunosuppression in the management of thyroid eye disease. Eye 11(Pt 5):717–722
Palmer D, Greenberg P, Cornell P, Parker RG (1987) Radiation therapy for Graves’ ophthalmopathy: a retrospective analysis. Int J Radiat Oncol Biol Phys 13(12):1815–1820
Zhang S, Wang Y, Zhong S, Liu X, Huang Y, Fang S et al (2018) Orbital radiotherapy plus three-wall orbital decompression in a patient with rare ocular manifestations of thyroid eye disease: case report. BMC Endocr Disord 18(1):7
Hersh D, Kinnar M (2014) Acute dysthyroid optic neuropathy exacerbated by orbital radiotherapy. Orbit 33(5):385–387
Rush S, Winterkorn JM, Zak R (2000) Objective evaluation of improvement in optic neuropathy following radiation therapy for thyroid eye disease. Int J Radiat Oncol Biol Phys 47(1):191–194
Threlkeld A, Miller NR, Wharam M (1989) The efficacy of supervoltage radiation therapy in the treatment of dysthyroid optic neuropathy. Orbit 8(4):253–264. https://doi.org/10.3109/01676838909012334
Trobe JD, Glaser JS, Laflamme P (1978) Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol 96(7):1199–1209
Hurbli T, Char DH, Harris J, Weaver K, Greenspan F, Sheline G (1985) Radiation therapy for thyroid eye diseases. Am J Ophthalmol 99(6):633–637
Kim JW, Han SH, Son BJ, Rim TH, Keum KC, Yoon JS (2016) Efficacy of combined orbital radiation and systemic steroids in the management of Graves’ orbitopathy. Graefe’s Arch Clin Exp Ophthalmol. 254(5):991–998
Shams PN, Ma R, Pickles T, Rootman J, Dolman PJ (2014) Reduced risk of compressive optic neuropathy using orbital radiotherapy in patients with active thyroid eye disease. Am J Ophthalmol 157(6):1299–1305
Author information
Authors and Affiliations
Contributions
Maryla Pelewicz-Sowa and Piotr Miśkiewicz drafted the manuscript and performed literature search and data analysis. Piotr Miśkiewicz had the idea for the article and critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pelewicz-Sowa, M., Miśkiewicz, P. Dysthyroid optic neuropathy: emerging treatment strategies. J Endocrinol Invest 46, 1305–1316 (2023). https://doi.org/10.1007/s40618-023-02036-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02036-0