Abstract
Purpose
Prepubescent body fat percentage (BFP) is associated with puberty onset; however, the association between the timing of puberty onset and BFP remains unclear. This study aimed to determine whether and how the timing of puberty onset is associated with various anthropometric measures, and to investigate the critical time period of the BFP transition before and after puberty.
Methods
The Taiwan Pubertal Longitudinal Study (TPLS) has a multicenter, population-based prospective cohort and was established in July 2018 at 4 pediatric departments. We included girls aged 6–14 years and boys aged 9–17 years evaluated as having puberty onset and excluded those with precocious puberty diagnosis. The anthropometric measures were collected every 3 months. The main outcome was age at puberty onset. Data were analyzed between July 2018 and September 2020.
Results
For 153 girls and 83 boys, BFP was significantly related to puberty onset for girls. Longitudinal analysis revealed that BFP in the girls was reduced to less than 18% 6 months before puberty and rapidly increased by 2.85% over 3 months, then exceeding 20% before puberty onset. After puberty onset, BFP was no longer lower than 22%.
Conclusions
BFP is an essential predictor of age at puberty onset. BFP first decreases and then begins to increase 3–6 months before puberty in girls. Parents and schools could monitor the BFP of prepubescent girls every 6 months to predict puberty onset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In prepubescent children, body fat percentage (BFP) is highly correlated to puberty onset [1]. In the 1980s, Frisch et al. hypothesized that a critical BFP is required for puberty onset [2]. Menarche is associated with the attainment of the average critical body weight for height, representing a critical body composition for BFP [2]. For girls with primary amenorrhea, a BFP of 17% is required for the onset of their menses. In those with secondary amenorrhea, a BFP of 22% is required for the restoration of their menses [3]. These critical BFPs imply that a particular body composition is essential for the onset and maintaining the pubertal process [3].
In accordance with Frisch’s hypothesis, many studies have discussed the applicability of critical weight for individuals and the methods used to estimate BFP [4,5,6]. Johnston et al. [5] reported that 8 girls reached menarche with the body weight of approximately 71.4 kg, indicating that Frisch’s concept of critical weight could not be applied to individuals. However, the authors observed a reduction in variability by estimating total body water instead of body weight. Crawford and Osler [4] suggested that menarche does not necessarily come after achieving a critical body weight or after a reduction in metabolism. They proposed that girls with gonadal dysgenesis or hypothyroidism were counterexamples of Frisch’s hypothesis. However, they indicated that menarche is highly correlated with the achievement of characteristic body composition when the ovaries are competent. Scott and Johnston [6] examined the methodology used to measure body fatness. In the 1980s, BFP was estimated using only height and weight. In this estimation, BFP can be transcribed as a bivariate function containing only body weight and height, which were considerably erroneous for individuals [6]. They concluded that the critical weight (fat) hypothesis could not be accepted based on available evidence and a hypothesis based on the central nervous system could provide a better explanation.
Body composition analyzers are widely used to directly measure BFP instead of estimating BFP by using body weight and total body water. A study found molecules that act as a message bridge between body fat and the central nervous system [7]. The majority of studies have indicated that puberty is correlated with the achievement of characteristic body composition in relatively healthy individuals. Hence, closely monitoring BFP as a determinant of puberty timing can be valuable in clinical practice. A well-designed longitudinal study closely monitoring BFP before and after puberty through direct measurement in the general population without endocrine disorders is warranted.
This study aimed to (1) analyze the timing of puberty onset and its association with multiple anthropometric measures, (2) predict the timing of puberty onset based on BFP by using a machine learning technique, and (3) perform a longitudinal assessment of BFP every 3 months both before and after puberty onset.
Methods
Study design and settings
This study used data from the Taiwan Pubertal Longitudinal Study (TPLS). The TPLS follows a multicenter, population-based, prospective cohort. Since July 2018, we have conducted the TPLS by recruiting girls aged between 6 and 14 years and boys aged between 9 and 17 years at pediatric endocrinology outpatient clinics at Taiwan Medical University Hospital, Taipei Municipal Wanfang Hospital, Cathay General Hospital (CGH), and National Cheng Kung University Hospital (NCKUH). This study was approved by the Institutional Review Board of Taipei Medical University (N201802018), CGH (CGH-P108107), and NCKUH (B-BR-108-076) and complied with the principles outlined in the Helsinki Declaration (Supplemental Materials: Taiwan Puberty Longitudinal Study).
Measurement of the timing of puberty onset
The main outcome variable was sexual maturation, which was evaluated every 3 months following enrollment. Tanner’s pubertal scale was used by pediatric endocrinologists at the outpatient clinic during each visit to determine the overall Tanner stage, the breast development for girls (staged as B1-B5) and the genital development for boys (staged as G1-G5) [8]. The development of the genitals for boys were also examined by measuring the testis volume (Tvol) [9]. We defined the timing of puberty onset as the month at which the participants achieved Tanner stage 2 for both sexes or achieved stage B2 (thelarche) for girls or testis volume ≥ 4 ml (the onset of testicular enlargement) for boys.
Anthropometric measures
The primary exposure variables were anthropometric measures collected every 3 months since enrollment; these were body height, body weight, waist circumference, body composition analysis results, and the findings of questionnaires on physical activity.
Body mass index (BMI) was defined as the weight (in kilograms) divided by the square of height (in meters) and then transformed into age- and sex-specific Z scores (zBMI) according to the World Health Organization (WHO) Child Growth Standards for school-aged children and adolescents [10, 11]. Waist circumference was measured in centimeters by using a flexible tape at the natural waist (midpoint between the lower ribcage and iliac crest) and recorded to the nearest 1 mm. At the level of the maximal posterior extension of the buttocks, hip circumference was measured in centimeters. The waist-to-height ratio was calculated as waist circumference divided by height, and the waist-to-hip ratio was calculated as waist circumference divided by hip circumference [11]. Body composition, including BFP and fat-free mass (FFM), was measured in light clothing by using a portable bioimpedance analysis (BIA) electronic scale TT-BC418 (Tanita Corp., Japan). BIA is considered a reliable and practical tool to estimate BFP in children [12]. The children were asked to avoid fluid or food intake and vigorous exercise 2 h prior to BIA measurements. In addition, BIA measurements vary among different ethnic groups, and our BIA machine used predictive equations adjusted for the Asian population [13]. Standardized protocols were used in every participating hospital to prevent systematic bias.
Measurement of confounding variables
The a priori confounding factors were prenatal factors (birth weight), postnatal factors (breastfeeding, diet composition and physical activity), and socioeconomic factors (parental education and family income) [14]. Breastfeeding was considered if children had been breastfed for at least 3 months after birth. Children were invited to record all instances of eating in written food records. Two trained dietitians independently disaggregated the foods into their constituent ingredients. The energy was averaged from the collected three-day data and calculated as kcal/day. Macronutrients include protein, fat, carbohydrates, and dietary fiber, and their basic units were calculated as grams [15]. The Chinese version of the International Physical Activity Questionnaire was used to determine the participants’ physical activity [16]. We calculated the participants’ metabolic equivalent values (METs) according to their physical activity in the past 7 days, and the physical activity level was classified into the following 3 categories: mild (< 3 kcal kg−1 h−1), moderate (3–6 kcal kg−1 h−1), and vigorous intensity (> 6 kcal kg−1 h−1) [16].
Inclusion and exclusion criteria of participants
In the TPLS cohort, we included girls aged between 6 and 14 years and boys aged between 9 and 17 years who visited the pediatric endocrinology clinic for pubertal growth assessment. Among these children, we included those evaluated as having Tanner stage 2, breast stage 2, or testis volume ≥ 4 ml during at least one visit until the end of September 2020. [17] The reason for the inclusion is that, the first physical marker for the onset of puberty in girls is typically the transition from Tanner breast stage B1 to B2, which includes the early growth of the breast tissue. In boys, this marker is the change from Tanner genital stage G1 to stage G2, including the enlargement of the testis [17]. We reviewed several definitions of testicular volume cut-points representing the onset of puberty in boys and adopted the achievement of a testicular volume of ≥ 4 ml [18]. We excluded children with precocious puberty diagnosis and those without adiposity measures. According to a previous study [19], the proportion of R2 for both BFP and Tanner stage can be approximately 0.11. Hence, the sample size calculated at a 5% significant level and 80% power was 122 participants in both the case and control groups.
Statistical analysis
To investigate critical variables related to the timing of puberty onset, we calculated the regression coefficient (β) of age at the month of puberty onset (years, months) for each anthropometric measure (%, namely zBMI, BFP, FFM percent, waist-to-hip ratio, and waist-to-height ratio), and parental education, family income, breastfeeding, and physical activity were adjusted (Table 2).
To compare the importance of various anthropometric measures, determine the critical cutoff point of BFP for the timing of puberty, and understand the clinical applicability of anthropometric measures when combined with the radiographic examination, we used the classification and regression tree (CART) to investigate the association between the anthropometric measures (input) and age at puberty onset (target). The CART model derived the cutoff points of these predictors. In addition, the difference between bone age and chronological age (BA-CA) was included as an input factor (Fig. 2; details are in the Supplemental Materials). We used the data of the participants from NCKUH as a validation set to determine the model’s accuracy.
To investigate the critical time period of the BFP transition before and after puberty, longitudinal measures were evaluated at 3 months intervals. We used months before and after the onset of puberty instead of chronological age to determine the association of time to puberty onset with change in BFP. The month in which the participants achieved Tanner stage 2 was considered the time of puberty onset, and the month in which the participants achieved thelarche was used in the sensitivity test. Thelarche (the onset of breast development, Tanner breast scale 2, B2) often indicates the beginning of pubertal development and is related to the reactivation of the hypothalamic–pituitary–gonadal (HPG) axis [17]. We use the mean to impute the missing data of anthropometric measures individually. Statistical analyses were conducted using R software (Version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Over 1500 children were enrolled in the TPLS until the end of September 2020, and 270 children met the inclusion criteria (Fig. 1). After excluding children with precocious puberty diagnosis (n = 27) and children without adiposity measures (n = 7), a total of 236 children (153 girls and 83 boys) were included for further analysis. The average follow-up period was 6.0 ± 5.8 months.
Table 1 lists the baseline characteristics of the study participants. The number of girls was nearly twice that of boys, and the average ages were 10.2 and 12.3 years, respectively. The parents of 90% of the children were highly educated (college or above), most of them came from well-off families (monthly household income over NT$100 000), and more than 80% of the children were breastfed for at least 3 months. Furthermore, 14% of the girls and 19.8% of the boys were overweight, and 15.4% of the girls and 22.2% of the boys were obese. 82% of girls and 55.4% of the boys had reached Tanner stage 2 at enrollment.
Critical anthropometric measures for puberty onset
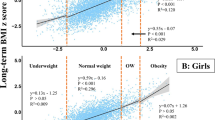
zBMI was significantly related to puberty onset for the girls (Table 2). Higher zBMI was associated with earlier puberty onset (P < 0.001). However, zBMI is not a comprehensive indicator of fatness in prepubescent children [20]. The findings of body composition analysis revealed that BFP and FFM were significantly related to the age at puberty onset in the girls (Table 2, P = 0.04, adjusted for parental education, family income, breastfeeding, and physical activity). We found that 1% higher BFP was correlated with 0.02 years (7.3 days) earlier puberty onset (Tanner 2) in the girls. The relationship between BFP and the age at which thelarche was achieved in the sensitivity test was also significant (Table 2, P = 0.02). However, due to the insufficient number of the included boys, the relationship between anthropometric measures and the timing of puberty onset in boys cannot be determined. Overall, the findings indicate that BFP and FFM of girls were significant anthropometric predictors for the timing of puberty onset.
Critical BFP for puberty onset
Figure 2 presents the final CART model for the training dataset. We found that bone age was the primary predictor of age at puberty onset; girls with more advanced bone age had earlier puberty onset. BFP was the second predictor of age at puberty onset. Among girls whose bone ages exceeded their chronological ages by 0.53–1.65 years, those with higher BFP (over 25.25%) tended to have puberty onset 1.05 years earlier than leaner girls.
The CART model revealed the importance and cutoff points of these predictors. Participants with bone age exceeding 0.53–1.65 years were segregated based on their BFP. Eight participants with BFP more than 25.25% had earlier ages at puberty onset (Fig. 2, node 5, mean puberty age = 9.34 years, standard error = 5.5) than did those with BFP less than 25.25% (Fig. 2, node 6, n = 16, mean puberty age = 10.39 years, standard error = 11.2). Overall, the final model’s first and second splits were defined with respect to the difference between BA-CA, and the third split was segregated with BFP as the predictor (details are in the Supplemental Materials: Machine learning).
The model has an accuracy of 0.75. The results of the multiple linear regressions and the relative variable importance method were similar to those of CART, and the accuracy of the validation set was satisfactory. Advanced bone age and BFP had higher importance than other parameters as determined by a relative importance ranking algorithm and were thus deemed essential parameters for predicting age at puberty onset. In the partial dependence analysis of BFP, a negative correlation was found if BFP was greater than 25% (details are in the Supplemental Materials).
Longitudinal change in BFP before and after puberty onset
We examined BFP every 3 months both before and after puberty during follow-up. We observed substantially different trends of BFP changes between boys and girls (Figure S1). For the girls, we found that mean BFP increased over time and reached 21.2% at the month of puberty onset. Boys had larger variations in BFP, and no apparent trend was found before or after puberty onset.
The regression plot and descriptive statistics with 3 months per unit are presented in Fig. 3 and Table S1, respectively. We observed that mean BFP did not always increase before puberty onset; instead, it first decreased and then increased. We noted that BFP rapidly increased 3–6 months before puberty (Fig. 3, + 2.85% in 3 months).
The results indicated that BFP 6 months before puberty is critical for the following puberty onset. At this time, BFP may decrease to a minimum and then rebound. The results of the paired t test revealed, that among time periods surrounding puberty onset, mean BFP significantly differed only during the 6 months prior to puberty onset, indicating that this period is a minimum for BFP (Table S2). Mean BFP decreased to < 18% 6 months before puberty onset and then increased to exceed 20%; puberty onset occurred 3 months later. After the onset of puberty, the mean BFP was no longer less than 22% (Fig. 3, Table S1).
In the sensitivity test, we found a nadir for BFP 9 months before thelarche and an increase in BFP from 9 to 3 months before thelarche (Fig. 3). BFP reached a minimum and rebounded during this period (+ 5.63% in 6 months); this was also the critical period for puberty onset.
Discussion
Summary of evidence
We observed that each 1% increase in BFP was correlated with 0.02 years (7.3 days) earlier puberty onset (Tanner 2) in the girls. The CART algorithm revealed that BFP was the second predictor of age at puberty onset in girls. Among girls whose bone age exceeded the chronological age of 0.53–1.65 years, those with higher BFP (over 25.25%) experienced puberty onset 1.05 years earlier than leaner girls.
We observed that BFP reached a minimum and then increased between 9 and 3 months before puberty onset. BFP in prepubescent girls reached a minimum of less than 18% (17.6% in this study) between 6 and 9 months before puberty onset, then increased to over 20% 3 months before puberty onset, and finally reached an average of 21.2% at the month of puberty onset. After the onset of puberty, BFP was no longer less than 22%; this result was in accordance with the minimum BFP of 22% required to maintain the menstrual cycle, as proposed by Frisch et al. Due to the insufficient numbers of boys, no relationship between BFP and puberty can be determined.
Interpretation
-
1.
Supportive evidence of the Frisch hypothesis
The results of this study supported the hypothesis of Frisch that BFP in girls is critical for the timing of puberty onset. A quantitative description was made based on the longitudinal cohort follow-up and extended to clinical decision-making and public health applications.
Frisch proposed that BFP less than 17% increased the risk of primary amenorrhea, and BFP less than 22% after menarche may cause secondary amenorrhea [2]. The results for our cohort were consistent with Frisch’s proposal. The nadir of BFP before puberty was not less than 17%, and BFP after puberty was not less than 22%. However, on the basis of the longitudinal results, we speculated that the key indicator of puberty onset could be the rebounding trend of increasing BFP rather than a critical BFP value.
-
2.
Rebounding trends and exceeded threshold of BFP-initiated puberty instead of high levels
Although a threshold level of leptin may be required for the initiation of puberty, an increase in leptin level does not appear to be the triggering event for the onset of puberty [1]. This finding might be attributable to leptin insensitivity induced by obesity through receptor downregulation [21, 22]. In our study, puberty occurred after the BFP rebounded and exceeded 20%; thus, we speculated that the trend of prepubescent BFP is the key factor for initiating puberty instead of a high body fat level during the prepubescent period. A recent study [23] revealed that higher prepubescent BFP is associated with earlier puberty but puberty timing is not associated with post-pubertal BFP change.
-
3.
Strengths
To our knowledge, no study has used machine learning to investigate how body composition affects puberty onset in children. In addition, this is the first study to examine long-term data regarding the body composition of school-aged children and to perform intensive follow-ups every 3 months. In contrast to the Frisch hypothesis, this study used a body composition analyzer to directly measure BFP rather than estimating it based on body weight and total body water.
This study elaborated the Frisch–Revelle hypothesis and provided evidence for the hypothesis for a healthy population. We found that the BFP of the girls reached a minimum and then rebounded between 6 and 9 months prior to puberty onset and then increased until puberty onset. This is the first study describing this critical period and thus providing evidence of the subtle intuition for some clinical pediatricians.
-
4.
Implications
Changes in BFP shifted from negative to positive 3–6 months prior to puberty onset in the girls (Fig S2). During this period, BFP initially decreased and then increased. Parents can measure the BFP of their prepubescent girls every semester and record the differences between each semester. If BFP begins to increasing after decreasing, puberty may begin in the next 6–9 months. These results are not extendable to pathologic conditions.
For public health, the addition of body composition screening to health checks of elementary school students can help prevent obesity and predict the timing of puberty onset in normal development girls. A body composition analyzer cost approximately NT$300,000, or US$9430. A school with 500 female students can perform a total of 6000 screenings in 6 years if measured once per semester, that is, the cost of each measurement is only NT$50, or US$1.57. If further cost savings are required, one machine could be shared among multiple schools. The machine can also be used as a follow-up tool for children with overweight or obesity, 21.3% of girls (106.5 girls) could benefit from its use [24].
Increased incidence of accelerated puberty in females was reported during the COVID-19 pandemic [25]. Our study period did not include the COVID-19 pandemic, however, children may have sedentary lifestyle and increased BFP during the quarantine period, which may advance the rebound of BFP and the puberty.
-
5.
Limitations
This study has some limitations that should be addressed. First, we cannot rule out the possibility of selection bias. The children were brought to the clinic for consultations for growth issues. Although we excluded participants with diseases, we still did not perform a random sampling of a healthy population. In addition, families in nearby areas have relatively high incomes, and their parents are almost all highly educated; hence, the findings may not be generalizable to a general population. However, we used data from individuals in southern Taiwan to further test our prediction model, and the results indicated that the model was moderately accurate. Another limitation was that the boy sample was smaller than the estimated 122, thus the relationship between the BFP and puberty cannot be determined.
Conclusion
BFP is an essential predictor of age at puberty onset. BFP first decreases and then increases between 3 and 6 months prior to puberty onset in girls. Parents and schools could closely monitor the BFP of prepubescent girls every 6 months to predict the timing of their puberty onset.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- BFP:
-
Body fat percentage
- T2:
-
Tanner stage 2
- B2:
-
Tanner scale breast stage 2
- G2:
-
Tanner scale genital stage 2
- Tvol:
-
Testis volume
- SE:
-
Standard error
- CI:
-
Confidence interval
References
Kaplowitz PB (2008) Link between body fat and the timing of puberty. Pediatrics 121(Suppl 3):S208–S217
Frisch RE, McArthur JW (1974) Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185(4155):949–951
Baker ER (1985) Body weight and the initiation of puberty. Clin Obstet Gynecol 28(3):573–579
Crawford JD, Osler DC (1975) Body composition at menarche: the Frisch-Revelle hypothesis revisited. Pediatrics 56(3):449–458
Johnston FE et al (1975) Critical weight at menarche. Critique of a hypothesis. Am J Dis Child 129(1):19–23
Scott EC, Johnston FE (1982) Critical fat, menarche, and the maintenance of menstrual cycles: a critical review. J Adolesc Health Care 2(4):249–260
Abreu AP, Kaiser UB (2016) Pubertal development and regulation. Lancet Diabetes Endocrinol 4(3):254–264
Tanner JM (1986) Normal growth and techniques of growth assessment. Clin Endocrinol Metab 15(3):411–451
Prader A (1966) Testicular size: assessment and clinical importance. Triangle 7(6):240–243
de Onis M, Habicht JP (1996) Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr 64(4):650–658
Nishida C, Ko GT, Kumanyika S (2010) Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr 64(1):2–5
Orta Duarte M et al (2014) Correlation between percentage of body fat measured by the Slaughter equation and bio impedance analysis technique in Mexican schoolchildren. Nutr Hosp 29(1):88–93
Haroun D et al (2010) Validation of bioelectrical impedance analysis in adolescents across different ethnic groups. Obesity (Silver Spring) 18(6):1252–1259
Yermachenko A, Dvornyk V (2014) Nongenetic determinants of age at menarche: a systematic review. Biomed Res Int 2014:371583
Wang JS et al (2019) Evaluation of a technological image-based dietary assessment tool for children during pubertal growth: a pilot study. Nutrients. https://doi.org/10.3390/nu11102527
Macfarlane DJ et al (2007) Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport 10(1):45–51
Howard SR, Dunkel L (2019) Delayed puberty-phenotypic diversity, molecular genetic mechanisms, and recent discoveries. Endocr Rev 40(5):1285–1317
Ma HM et al (2011) Pubertal development timing in urban Chinese boys. Int J Androl 34(5 Pt 2):e435–e445
Fan HY et al (2020) Body mass index growth trajectories, early pubertal maturation, and short stature. Pediatr Res 88(1):117–124
Wilkes M et al (2019) Relationship of BMI z score to fat percent and fat mass in multiethnic prepubertal children. Pediatr Obes. https://doi.org/10.1111/ijpo.12463
Mitchell SE et al (2009) Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol 587(Pt 14):3573–3585
Tups A et al (2017) Central regulation of glucose homeostasis. Compr Physiol 7(2):741–764
O’Keeffe LM et al (2020) Puberty timing and adiposity change across childhood and adolescence: disentangling cause and consequence. Hum Reprod 35(12):2784–2792
Chu NF (2005) Prevalence of obesity in Taiwan. Obes Rev 6(4):271–274
Chioma L et al (2022) Sedentary lifestyle and precocious puberty in girls during the COVID-19 pandemic: an Italian experience. Endocr Connect. https://doi.org/10.1530/EC-21-0650
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan, grant number MOST 107-2314-B-038-113-MY3, MOST 110-2628-B-038-014, 111TMUH-MOST-05 and MOST 111-2628-B-038-022, the Cathay General Hospital (CGH), grant number 109CGH-TMU-04, the Higher Education Sprout Project of the Ministry of Education (MOE), grant number DP2-110-21121-01-A-04, and the Taipei Medical University Hospital (TMUH), grant number 111TMUH-NE-02.
Author information
Authors and Affiliations
Contributions
YCC. conceived the original idea and presided over the project. J-WH, M-CT, CY, and YCC enrolled the participants. LH, H-YF, and JB performed the analysis. J-WH and YCC verified analytical methods and supervised the findings of this work. LH wrote the manuscript with support from J-WH and Y-CC All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All samples in TPLS have been collected and utilized following strict human subject protection guidelines and Institutional Review Board review of protocols.
Informed consent
All samples in TPLS have been collected and utilized following informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, L., Hou, JW., Fan, HY. et al. Critical body fat percentage required for puberty onset: the Taiwan Pubertal Longitudinal Study. J Endocrinol Invest 46, 1177–1185 (2023). https://doi.org/10.1007/s40618-022-01970-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01970-9