Abstract
Purpose
Fine and balanced regulation of cell proliferation and apoptosis are key to achieve ovarian follicle development from the primordial to the preovulatory stage and therefore assure female reproductive function. While gonadotropins are the major and most recognized regulators of follicle cell growth and function, other factors, both systemic and local, play equally important roles. This work is aimed at evaluating the effects of thyroid hormones (THs) on human granulosa luteinized (hGL) viability.
Methods
Human GL cells derived from assisted reproduction treatments were exposed to T3 or T4. Cell viability was evaluated by MTT assay. Apoptosis was evaluated by the TUNEL assay and active caspase-3 staining. StAR, CYP19A1,Caspase-3, P53 and BAX mRNA were evaluated by real-time PCR. LC3-I/-II, AKT and pAKT were evaluated by western blot.
Results
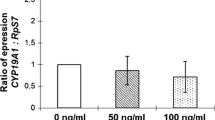
T3 and T4 promoted cell viability in a dose-dependent modality and modulate StAR and CYP19A1 expression. T3 and to a lesser extent T4 mitigated cell death induced by serum starvation by inhibition of caspase-3 activity and expression of P53 and BAX; and attenuate cell death experimentally induced by C2-ceramide. Cell death derived from starvation appeared to be involved in autophagic processes, as the levels of autophagic markers (LC3-II/LC3-I ratio) decreased when starved cells were exposed to T3 and T4. This effect was associated with an increase in pAkt levels.
Conclusion
From the present study, THs emerge as potent anti-apoptotic agents in hGL cells. This effect is achieved by inhibiting the apoptosis signalling pathway of BAX and caspase-3, while maintaining active the PI3K/AKT pathway.










Similar content being viewed by others
References
McGee EA, Hsueh AJ (2000) Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21(2):200–214. https://doi.org/10.1210/edrv.21.2.0394
Matsuda F, Inoue N, Manabe N et al (2012) Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 58(1):44–50. https://doi.org/10.1262/jrd.2011-012
Jiang JY, Cheung CK, Wang Y et al (2003) Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci 8:d222–d237. https://doi.org/10.2741/949
Palumbo A, Yeh J (1995) Apoptosis as a basic mechanism in the ovarian cycle: follicular atresia and luteal regression. J Soc Gynecol Investig 2(3):565–573. https://doi.org/10.1177/107155769500200310
Tilly JL, Kowalski KI, Johnson AL et al (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129(5):2799–2801. https://doi.org/10.1210/endo-129-5-2799
Canipari R (2000) Oocyte–granulosa cell interactions. Hum Reprod Update 6(3):279–289. https://doi.org/10.1093/humupd/6.3.279
Wakim AN, Polizotto SL, Buffo MJ et al (1993) Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril 59(6):1187–1190. https://doi.org/10.1016/S0015-0282(16)55974-3
Pascual A, Aranda A (2013) Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta 1830(7):3908–3916. https://doi.org/10.1016/j.bbagen.2012.03.012
Zhang C, Wang X, Wang Z et al (2013) Effect of different culture systems and 3, 5, 3′-triiodothyronine/follicle-stimulating hormone on preantral follicle development in mice. PLoS One 8(4):e61947. https://doi.org/10.1371/journal.pone.0061947
Zhang C, Guo L, Zhu B et al (2013) Effects of 3, 5, 3′-triiodothyronine (t3) and follicle stimulating hormone on apoptosis and proliferation of rat ovarian granulosa cells. Chin J Physiol 56(5):298–305. https://doi.org/10.4077/CJP.2013.BAB186
Fedail JS, Zheng K, Wei Q et al (2014) Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 46(3):594–604. https://doi.org/10.1007/s12020-013-0092-y
Poppe K, Velkeniers B, Glinoer D (2007) Thyroid disease and female reproduction. Clin Endocrinol (Oxf) 66(3):309–321. https://doi.org/10.1111/j.1365-2265.2007.02752.x
Dittrich R, Beckmann MW, Oppelt PG et al (2011) Thyroid hormone receptors and reproduction. J Reprod Immunol 90(1):58–66. https://doi.org/10.1016/j.jri.2011.02.009
Mintziori G, Anagnostis P, Toulis KA et al (2012) Thyroid diseases and female reproduction. Minerva Med 103(1):47–62
Cho MK (2015) Thyroid dysfunction and subfertility. Clin Exp Reprod Med 42(4):131–135. https://doi.org/10.5653/cerm.2015.42.4.131
Vissenberg R, Manders VD, Mastenbroek S et al (2015) Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update 21(3):378–387. https://doi.org/10.1093/humupd/dmv004
Duarte-Guterman P, Navarro-Martin L, Trudeau VL (2014) Mechanisms of crosstalk between endocrine systems: regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen Comp Endocrinol 203:69–85. https://doi.org/10.1016/j.ygcen.2014.03.015
Aghajanova L, Lindeberg M, Carlsson IB et al (2009) Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online 18(3):337–347. https://doi.org/10.1016/S1472-6483(10)60091-0
Kinne A, Schulein R, Krause G (2011) Primary and secondary thyroid hormone transporters. Thyroid Res 4(Suppl 1):S7. https://doi.org/10.1186/1756-6614-4-S1-S7
Canipari R, Mangialardo C, Di Paolo V et al (2018) Thyroid hormones act as mitogenic and pro survival factors in rat ovarian follicles. J Endocrinol Invest 42(3):271–282. https://doi.org/10.1007/s40618-018-0912-2
Verga-Falzacappa C, Timperi E, Bucci B et al (2012) T(3) preserves ovarian granulosa cells from chemotherapy-induced apoptosis. J Endocrinol 215(2):281–289. https://doi.org/10.1530/JOE-12-0153
Verga-Falzacappa C, Mangialardo C, Patriarca V et al (2009) Thyroid hormones induce cell proliferation and survival in ovarian granulosa cells COV434. J Cell Physiol 221(1):242–253. https://doi.org/10.1002/jcp.21849
Morelli MB, Barberi M, Gambardella A et al (2008) Characterization, expression, and functional activity of pituitary adenylate cyclase-activating polypeptide and its receptors in human granulosa-luteal cells. J Clin Endocrinol Metab 93(12):4924–4932. https://doi.org/10.1210/jc.2007-2621
Innocenti F, Cerquetti L, Pezzilli S et al (2017) Effect of mitotane on mouse ovarian follicle development and fertility. J Endocrinol 234(1):29–39. https://doi.org/10.1530/JOE-17-0203
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Perks CM, Newcomb PV, Grohmann M et al (2003) Prolactin acts as a potent survival factor against C2-ceramide-induced apoptosis in human granulosa cells. Hum Reprod 18(12):2672–2677. https://doi.org/10.1093/humrep/deg496
Samuels HH, Stanley F, Casanova J (1979) Relationship of receptor affinity to the modulation of thyroid hormone nuclear receptor levels and growth hormone synthesis by l-triiodothyronine and iodothyronine analogues in cultured GH1 cells. J Clin Invest 63(6):1229–1240. https://doi.org/10.1172/JCI109418
Kobayashi N, Orisaka M, Cao M et al (2009) Growth differentiation factor-9 mediates follicle-stimulating hormone-thyroid hormone interaction in the regulation of rat preantral follicular development. Endocrinology 150(12):5566–5574. https://doi.org/10.1210/en.2009-0262
Fitko R, Kucharski J, Szlezyngier B et al (1996) The concentration of GnRH in hypothalamus, LH and FSH in pituitary, LH, PRL and sex steroids in peripheral and ovarian venous plasma of hypo- and hyperthyroid, cysts-bearing gilts. Anim Reprod Sci 45(1–2):123–138. https://doi.org/10.1016/s0378-4320(96)01568-0
Maruo T, Hayashi M, Matsuo H et al (1987) The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology 121(4):1233–1241. https://doi.org/10.1210/endo-121-4-1233
Goldman S, Dirnfeld M, Abramovici H et al (1993) Triiodothyronine (T3) modulates hCG-regulated progesterone secretion, cAMP accumulation and DNA content in cultured human luteinized granulosa cells. Mol Cell Endocrinol 96(1–2):125–131. https://doi.org/10.1210/jcem.82.6.3997
Goldman S, Dirnfeld M, Abramovici H et al (1997) Triiodothyronine and follicle-stimulating hormone, alone and additively together, stimulate production of the tissue inhibitor of metalloproteinases-1 in cultured human luteinized granulosa cells. J Clin Endocrinol Metab 82(6):1869–1873. https://doi.org/10.1210/jcem.82.6.3997
Hatsuta M, Tamura K, Shimizu Y et al (2004) Effect of thyroid hormone on CYP19 expression in ovarian granulosa cells from gonadotropin-treated immature rats. J Pharmacol Sci 94(4):420–425. https://doi.org/10.1254/jphs.94.420
Cecconi S, Rucci N, Scaldaferri ML et al (1999) Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology 140(4):1783–1788. https://doi.org/10.1210/endo.140.4.6635
Hirata R, Hojo T, Sano M et al (2015) Potential role of hCG in apoptosis of human luteinized granulosa cells. J Reprod Dev 61(1):67–73. https://doi.org/10.1262/jrd.2014-115
Kim WG, Zhu X, Kim DW et al (2013) Reactivation of the silenced thyroid hormone receptor beta gene expression delays thyroid tumor progression. Endocrinology 154(1):25–35. https://doi.org/10.1210/en.2012
Choi J, Jo M, Lee E et al (2011) Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil Steril 95(4):1482–1486. https://doi.org/10.1016/j.fertnstert.2010.06.006
Choi J, Jo M, Lee E et al (2014) AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 147(1):73–80. https://doi.org/10.1530/REP-13-0386
Matikainen T, Perez GI, Zheng TS et al (2001) Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 142(6):2468–2480. https://doi.org/10.1210/endo.142.6.8078
Perez GI, Robles R, Knudson CM et al (1999) Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21(2):200–203. https://doi.org/10.1038/5985
Herr D, Keck C, Tempfer C et al (2004) Chorionic gonadotropin regulates the transcript level of VHL, p53, and HIF-2alpha in human granulosa lutein cells. Mol Reprod Dev 69(4):397–401. https://doi.org/10.1002/mrd.20137
Davis PJ, Leonard JL, Davis FB (2008) Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29(2):211–218. https://doi.org/10.1016/j.yfrne.2007.09.003
Funding
This study was supported by grants from Sapienza University of Rome, Ateneo Federato 2017–2018 (to R. C.) (Grand No.: Finanziamenti Ateneo per la ricerca 2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The research was carried out according to the principles of the Declaration of Helsinki and was approved by the local institutional review board.
Informed consent
Informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Paolo, V., Mangialardo, C., Zacà, C. et al. Thyroid hormones T3 and T4 regulate human luteinized granulosa cells, counteracting apoptosis and promoting cell survival. J Endocrinol Invest 43, 821–831 (2020). https://doi.org/10.1007/s40618-019-01169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01169-5