Abstract
Purpose
To determine the validity of a self-administered questionnaire (Acro-CQ) developed to systematically assess the presence, type and time of onset of acromegaly comorbidities.
Methods
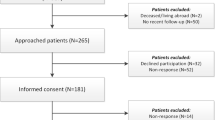
This is a cross-sectional study; 105 acromegaly patients and 147 controls with other types of pituitary adenoma, referred to a specialized Italian Center, autonomously compiled Acro-CQ in an outpatient clinical setting. To test its reliability in a different setting, Acro-CQ was administered via mail to 78 patients with acromegaly and 100 with other pituitary adenomas, referred to a specialized US Center. Data obtained from questionnaires in both settings were compared with medical records (gold standard).
Results
Demographics of patients and controls from both countries were similar. In both settings, >95 % of the questionnaires were completely filled; only one item was missed in the others. Concordance with medical record was excellent (k > 0.85) for most of the items, independently from the way of administration, patient age, gender and nationality, pituitary adenoma type and disease activity.
Conclusions
Acro-CQ is an inexpensive, highly accepted from patients and reliable tool recommended to expedite systematic collection of relevant clinical data in acromegaly at diagnosis, to be replicated at follow-ups. This tool may guide a targeted, cost-effective management of complications. Moreover, it could be applied to retrieve data for survey studies in both acromegaly and other pituitary adenomas, as information is easily and rapidly accessible for statistical analysis.


Similar content being viewed by others
References
Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J (2009) Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab 23:555–574
Daly AF, Beckers A (2015) Familial isolated pituitary adenomas (FIPA) and mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrinol Metab Clin North Am 44:19–25
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25:102–152
Jurcut R, Găloiu S, Florian A, Vlădaia A, Ioniţă OR, Amzulescu MS, Baciu I, Popescu BA, Cocolescu M, Ginghina C (2014) Quantifying subtle changes in cardiovascular mechanics in acromegaly: a Doppler myocardial imaging study. J Endocrinol Invest 37:1081–1090
Giustina A, Chanson P, Bronstein MD et al (2010) Acromegaly Consensus Group: a consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148
Melmed S, Casanueva FF, Hoffman AR et al (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288
Molitch ME (2014) Nonfunctioning pituitary tumors. Handb Clin Neurol 124:167–184
Giustina A, Chanson P, Kleinberg D et al (2014) Acromegaly Consensus Group: expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol 10:243–248
Melmed S, Casanueva FF, Klibanski A et al (2013) A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 16:294–302
Rydén L, Grant PJ, Anker SD et al (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34:3035–3087
Smith SC Jr, Grundy SM (2014) 2013 ACC/AHA guideline recommends fixed-dose strategies instead of targeted goals to lower blood cholesterol. J Am Coll Cardiol 64:601–612
Florence R, Allen S, Benedict L et al (2013) Diagnosis and treatment of osteoporosis. http://www.icsi.org/_asset/vnw0c3/Osteo.pdf. Accessed 12 July 2015
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Colosi L (2006) http://www.human.cornell.edu/pam/outreach/parenting/parents/upload/Designing-20an-20Effective-20Questionnaire.pdf. Accessed 12 July 2015
Burns KEA, Duffett M, Kho ME et al (2012) A guide for the design and conduct of sel-administered surveys of clinicians. Can Med Ass J 179:245–252
Lavelle K, Todd C, Campbell M (2008) Do postage stamps versus pre-paid envelopes increase responses to patient mail surveys? A randomized controlled trial. BMC Health Serv Res 8:113
Alexopoulou O, Bex M, Kamenicky P, Bessomo Mvoula A, Chanson P, Maiter D (2014) Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary 17:81–89
Mosca S, Paolillo S, Colao A et al (2013) Cardiovascular involvement in patients affected by acromegaly: an appraisal. Int J Cardiol 167:1712–1718
Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, Floriani I, Giustina A et al (2015) Bone turnover, bone mineral density and fracture risk in acromegaly: a metanalysis. J Clin Endocrinol Metab 100:384–394
Reverter JL, Fajardo C, Resmini E et al (2014) Benign and malignant nodular thyroid disease in acromegaly. Is a routine thyroid ultrasound evaluation advisable? PLoS One 9:e104174
Jenkins PJ (2006) Cancers associated with acromegaly. Neuroendocrinology 83:218–223
Wassenaar MJ, Cazemier M, Biermasz NR et al (2010) Acromegaly is associated with an increased prevalence of colonic diverticula: a case–control study. J Clin Endocrinol Metab 95:2073–2079
Attanasio R, Mainolfi A, Grimaldi F et al (2008) Somatostatin analogs and gallstones: a retrospective survey on a large series of acromegalic patients. J Endocrinol Invest 31:704–710
Ajmal A, Haghshenas A, Attarian S et al (2014) The effect of somatostatin analogs on vitamin D and calcium concentrations in patients withacromegaly. Pituitary 17:366–373. doi:10.1007/s11102-013-0514-0
Wolinski K, Czarnywojtek A, Ruchala M (2014) Risk of thyroid nodular disease and thyroid cancer in patients with acromegaly—meta-analysis and systematic review. PLoS One 9:e88787
Rokkas T, Pistiolas D, Sechopoulos P, Margantinis G, Koukoulis G (2008) Risk of colorectal neoplasm in patients with acromegaly: a meta-analysis. World J Gastroenterol 14:3484–3489
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RS serves in an advisory board for Novartis and receives research support from Novartis, Ipsen and Pfizer. EG serves in an advisory board for Pfizer. SG serves in an advisory board for Pfizer and received support from Novartis, Ipsen, Italfarmaco and Pfizer. The other authors declare they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the Helsinki Declaration. This article does not contain any studies with animals performed by any of the authors.
Informed consent
All patients included in the study gave their informed consent.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guaraldi, F., Gori, D., Beccuti, G. et al. Usefulness of an ad hoc questionnaire (Acro-CQ) for the systematic assessment of acromegaly comorbidities at diagnosis and their management at follow-up. J Endocrinol Invest 39, 1277–1284 (2016). https://doi.org/10.1007/s40618-016-0476-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-016-0476-y