Abstract
ᅟ
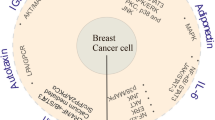
This review highlights the recent advances in our understanding of adipocyte contributions to carcinogenesis or cancer disease progression for cancers in the bone.
Purpose of Review
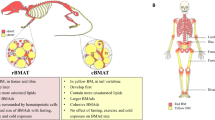
In this review, we aim to describe bone marrow adipose tissue and discuss the soluble adipocyte-derived cytokines (adipokines) or endocrine factors, adipocyte-derived lipids, and the actual or putative juxtacrine signaling between bone marrow adipocytes and tumor cells in the bone marrow. This relationship likely affects tumor cell initiation, proliferation, metastasis, and/or drug resistance.
Recent Findings
Bone marrow adipose may affect tumor proliferation, drug resistance, or cancer-induced bone disease and hence may be a new target in the fight against cancer.
Summary
Overall, evidence is mixed regarding the role of bone marrow adipose and adipocytes in cancer progression, and more research in this arena is necessary to determine how these bone marrow microenvironmental cells contribute to malignancies in the marrow to identify novel, potentially targetable pathways.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Beason TS, Chang S-H, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist. 2013;18:1074–9. https://doi.org/10.1634/theoncologist.2013-0015.
Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166:667–76. https://doi.org/10.1111/bjh.12935.
• Veld J, O’Donnell EK, Reagan MR, Yee AJ, Torriani M, Rosen CJ, et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skelet Radiol. 2016;45:1277–83. https://doi.org/10.1007/s00256-016-2425-4. This important research was the first to demonstrate a connection between abdominal adiposity and progression from MGUS to MM.
Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–74. https://doi.org/10.1158/0008-5472.CAN-09-0800.
Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–23. 10.18632/oncotarget.1482.
Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3:141–7. https://doi.org/10.1016/S2213-8587(14)70007-5.
Hudak CS, Gulyaeva O, Wang Y, Park S-M, Lee L, Kang C, et al. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–87. https://doi.org/10.1016/j.celrep.2014.06.060.
Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23:577–85. https://doi.org/10.1002/ajhb.21202.
Krishnamoorthy D, Frechette DM, Adler BJ, Green DE, Chan ME, Rubin CT. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos Int. 2015; https://doi.org/10.1007/s00198-015-3289-5.
Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. https://doi.org/10.1016/j.bone.2014.03.044.
de Araújo IM, Salmon CEG, Nahas AK, Nogueira-Barbosa MH, Elias J, de Paula FJA. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur J Endocrinol. 2017;176:21–30. https://doi.org/10.1530/EJE-16-0448.
Singh L, Brennan TA, Russell E, Kim J-H, Chen Q, Brad Johnson F, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29–36. https://doi.org/10.1016/j.bone.2016.01.014.
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–71.
Kretlow JD, Jin Y-Q, Liu W, Zhang WJ, Hong T-H, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. https://doi.org/10.1186/1471-2121-9-60.
Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle. 2010;9:3648–54. https://doi.org/10.4161/cc.9.18.13046.
•• Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82. https://doi.org/10.1182/blood-2011-01-330407. This groundbreaking finding was the first to show that adiponectin has anti-myeloma properties.
Falank C, Fairfield H, Reagan MR. Signaling interplay between bone marrow adipose tissue and multiple myeloma cells. Front Endocrinol (Lausanne). 2016;7:67. https://doi.org/10.3389/fendo.2016.00067.
McDonald MM, Fairfield H, Falank C, Reagan MR. Adipose, bone, and myeloma: contributions from the microenvironment. Calcif Tissue Int. 2016; https://doi.org/10.1007/s00223-016-0162-2.
Soley L, Falank C, Reagan MR. MicroRNA transfer between bone marrow adipose and multiple myeloma cells. Curr Osteoporos Rep. 2017;15:162–70. https://doi.org/10.1007/s11914-017-0360-5.
Trotter TN, Gibson JT, Sherpa TL, Gowda PS, Peker D, Yang Y. Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. Am J Pathol. 2016;186:3054–63. https://doi.org/10.1016/j.ajpath.2016.07.012.
Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329–41. 10.18632/oncotarget.6020.
• Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–4. https://doi.org/10.1038/sj.leu.2404658. This seminal piece of research first drew attention to the potential for bone marrow adipose tissue to contribute to myeloma.
• Yu W, Cao D-D, Li Q, Mei H, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7:86075–86. 10.18632/oncotarget.13342. This important manuscript demonstrates that leptin, derived from adipocytes, has tumor survival effects on myeloma cells.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. https://doi.org/10.1038/nm.2492.
Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GVN, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–9. https://doi.org/10.1038/leu.2014.112.
Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–5. https://doi.org/10.1038/nature22794.
Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer. 2010;102:403–13. https://doi.org/10.1038/sj.bjc.6605481.
Templeton ZS, Lie W-R, Wang W, Rosenberg-Hasson Y, Alluri RV, Tamaresis JS, et al. Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia. 2015;17:849–61. https://doi.org/10.1016/j.neo.2015.11.005.
•• Lwin ST, Olechnowicz SWZ, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10. https://doi.org/10.1038/leu.2014.295. This important manuscript demonstrates that obesity can induce greater myeloma survival and proliferation in the bone marrow and that this may be via increased IGF-1.
Sulston RJ, Learman BS, Zhang B, Scheller EL, Parlee SD, Simon BR, et al. Increased circulating adiponectin in response to Thiazolidinediones: investigating the role of bone marrow adipose tissue. Front Endocrinol (Lausanne). 2016;7:128. https://doi.org/10.3389/fendo.2016.00128.
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. https://doi.org/10.1016/j.cell.2010.02.027.
Messier TL, Boyd JR, Gordon JAR, Stein JL, Lian JB, Stein GS. Oncofetal epigenetic bivalency in breast cancer cells: H3K4 and H3K27 tri-methylation as a biomarker for phenotypic plasticity. J Cell Physiol. 2016;231:2474–81. https://doi.org/10.1002/jcp.25359.
Zych J, Stimamiglio MA, Senegaglia AC, Brofman PRS, Dallagiovanna B, Goldenberg S, et al. The epigenetic modifiers 5-aza-2′-deoxycytidine and trichostatin A influence adipocyte differentiation in human mesenchymal stem cells. Braz J Med Biol Res. 2013;46:405–16. https://doi.org/10.1590/1414-431X20132893.
Meyer MB, Benkusky NA, Sen B, Rubin J, Pike JW. Epigenetic plasticity drives Adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J Biol Chem. 2016;291(34):17829–47. https://doi.org/10.1074/jbc.M116.736538.
Fani N, Ziadlou R, Shahhoseini M, Baghaban EM. Comparative epigenetic influence of autologous versus fetal bovine serum on mesenchymal stem cells through in vitro osteogenic and adipogenic differentiation. Exp Cell Res. 2016;344:176–82. https://doi.org/10.1016/j.yexcr.2015.10.009.
Li G, Yao W, Jiang H. Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J Nutr. 2014;144:1887–95. https://doi.org/10.3945/jn.114.198531.
Rumberger JM, Arch JRS, Green A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. PeerJ. 2014;2:e611. https://doi.org/10.7717/peerj.611.
Bricambert J, Favre D, Brajkovic S, Bonnefond A, Boutry R, Salvi R, et al. Impaired histone deacetylases 5 and 6 expression mimics the effects of obesity and hypoxia on adipocyte function. Mol Metab. 2016;5:1200–7. https://doi.org/10.1016/j.molmet.2016.09.011.
Ali D, Alshammari H, Vishnubalaji R, Chalisserry EP, Hamam R, Alfayez M, et al. CUDC-907 promotes bone marrow Adipocytic differentiation through inhibition of histone deacetylase and regulation of cell cycle. Stem Cells Dev. 2017;26:353–62. https://doi.org/10.1089/scd.2016.0183.
Tabe Y, Yamamoto S, Saitoh K, Sekihara K, Monma N, Ikeo K, et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute Monocytic leukemia cells. Cancer Res. 2017;77:1453–64. https://doi.org/10.1158/0008-5472.CAN-16-1645.
Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–32. https://doi.org/10.1182/blood-2016-08-734798.
Reagan MR, Mishima Y, Glavey SV, Zhang YY, Manier S, Lu ZN, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124:3250–9. https://doi.org/10.1182/blood-2014-02-558007.
Glavey SV, Naba A, Manier S, Clauser K, Tahri S, Park J, et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia. 2017; https://doi.org/10.1038/leu.2017.102.
Bar-Natan M, Stroopinsky D, Luptakova K, Coll MD, Apel A, Rajabi H, et al. Bone marrow stroma protects myeloma cells from cytotoxic damage via induction of the oncoprotein MUC1. Br J Haematol. 2017;176:929–38. https://doi.org/10.1111/bjh.14493.
Fan Y, Bi R, Densmore MJ, Sato T, Kobayashi T, Yuan Q, et al. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2015; https://doi.org/10.1096/fj.15-278184.
Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289:16699–710. https://doi.org/10.1074/jbc.M114.547919.
Fan Y, Hanai J, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017;25:661–72. https://doi.org/10.1016/j.cmet.2017.01.001.
Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. https://doi.org/10.1038/ncb3570.
Lawson MA, McDonald MM, Kovacic NN, Khoo WH, Terry RTL, Down J, et al. Osteoclasts control re-activation of dormant myeloma cells by remodeling the endosteal niche. Nat Commun. 2015;6:8983. https://doi.org/10.1038/ncomms9983.
Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76:1089–100. https://doi.org/10.1158/0008-5472.CAN-15-1703.
Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–401. https://doi.org/10.1038/leu.2011.381.
Trotter TN, Fok M, Gibson JT, Peker D, Javed A, Yang Y. Osteocyte apoptosis attracts Myeloma cells to bone and supports progression through regulation of the bone marrow microenvironment. ASH Annu. Meet. Abstr., San Diego, CA: ASH Oral Presentation #484. 2016, p. Session: 651. Myeloma: Biology and Pathophysiology.
McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, et al. Inhibiting the osteocyte specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017; https://doi.org/10.1182/blood-2017-03-773341.
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2017; https://doi.org/10.1002/jcp.25976.
Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. https://doi.org/10.1111/nyas.12327.
Suchacki KJ, Cawthorn WP, Rosen CJ. Bone marrow adipose tissue: formation, function and regulation. Curr Opin Pharmacol. 2016;28:50–6. https://doi.org/10.1016/j.coph.2016.03.001.
Fairfield H, Rosen CJ, Reagan MR. Connecting bone and fat: the potential role for sclerostin. Curr Mol Biol Reports. 2017;3:114–21. https://doi.org/10.1007/s40610-017-0057-7.
Balani DH, Ono N, Kronenberg HM. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Invest. 2017; https://doi.org/10.1172/JCI91699.
Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J Endocrinol. 2017;232:461–74. https://doi.org/10.1530/JOE-16-0484.
Xu J-C, Wu G-H, Zhou L-L, Yang X-J, Liu J-T. Leptin improves osteoblast differentiation of human bone marrow stroma stem cells. Eur Rev Med Pharmacol Sci. 2016;20:3507–13.
Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–76. https://doi.org/10.1002/jcp.20804.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. https://doi.org/10.1038/nature08099.
Kawano Y, Roccaro A, Azzi J, Ghobrial I. Multiple myeloma and the immune microenvironment. Curr Cancer Drug Targets. 2017;17:1–1. https://doi.org/10.2174/1568009617666170214102301.
Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, et al. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta. 1846;2014:26–39. https://doi.org/10.1016/j.bbcan.2014.03.005.
Glavey SV, Manier S, Natoni A, Sacco A, Moschetta M, Reagan MR, et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014;124:1765–76. https://doi.org/10.1182/blood-2014-03-560862.
Moschetta M, Mishima Y, Kawano Y, Manier S, Paiva B, Palomera L, et al. Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia. 2016;30:1103–15. https://doi.org/10.1038/leu.2016.3.
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. https://doi.org/10.1158/0008-5472.CAN-10-3323.
Clark R, Krishnan V, Schoof M, Rodriguez I, Theriault B, Chekmareva M, et al. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183:576–91. https://doi.org/10.1016/j.ajpath.2013.04.023.
Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes Adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18:782–96. https://doi.org/10.1016/j.stem.2016.02.015.
• Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise decreases marrow adipose tissue though ß-oxidation in obese running mice. J Bone Miner Res. 2017; https://doi.org/10.1002/jbmr.3159. This excellent work demonstrates that bone marrow adipose can be targeted with exercise, suggesting non-pharmacological ways to modulate bone marrow adipose tissue and providing new persepctives of how and why exercise may be so beneficial.
Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise regulation of marrow fat in the setting of PPARγ agonist treatment in female C57BL/6 mice. Endocrinology. 2015;156:2753–61. https://doi.org/10.1210/en.2015-1213.
Acknowledgements
The authors’ work is supported by MMCRI Start-up funds, a pilot project grant from NIH/NIGMS (P30GM106391), the NIH/NIDDK (R24DK092759-01), and the COBRE grant from the NIH/NIGMS (P20GM121301). This work was also funded in part by pilots from NIGMS/NIH P30 GM106391 and the American Cancer Society (Research Grant #IRG-16-191-33). This review was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30AR066261. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carolyne Falank, Heather Fairfield, and Michaela R. Reagan each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Bone Metastasis
Rights and permissions
About this article
Cite this article
Falank, C., Fairfield, H. & Reagan, M.R. Reflections on Cancer in the Bone Marrow: Adverse Roles of Adipocytes. Curr Mol Bio Rep 3, 254–262 (2017). https://doi.org/10.1007/s40610-017-0074-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-017-0074-6