Abstract
Background
Preclinical studies have shown a therapeutic role of the mechanistic/mammalian target of rapamycin complex 1 (mTORC1) inhibition with rapamycin and its analogues (rapalogues) on several age-related musculoskeletal disorders (MSKD). However, the applicability to humans of these findings is unknown.
Objective
To assess the efficacy of rapalogues on age-related MSKD in humans.
Methods
We conducted a systematic review according to the PRISMA guidelines. MEDLINE, EMBase, EMCare, and Cochrane Central Registry of Controlled Trials were searched for original studies examining the effects of rapalogues on outcomes linked to the age-related MSKD in humans. This review is registered in the PROSPERO database (University of New York; registration number CRD42020208167).
Results
Fourteen studies met the inclusion criteria and were analyzed. The effect of rapamycin and other rapalogues, including everolimus and temsirolimus, on bone, muscle and joints have been evaluated in humans; however, considerable variability concerning the subjects’ age, inclusion criteria, and drug administration protocols was identified. In bone, the use of rapamycin is associated with a decrease in bone resorption markers dependent on osteoclastic activity. In muscle, rapamycin and rapalogues are associated with a reduction in muscle protein synthesis in response to exercise. In the context of rheumatoid arthritis, rapamycin and rapalogues have been associated with clinical improvement and a decrease in inflammatory activity.
Conclusion
Although there are studies that have evaluated the effect of rapamycin and rapalogues on MSKD in humans, the evidence supporting its use is still incipient, and the clinical implication of these results on the development of osteoporosis, sarcopenia, or osteosarcopenia has not been studied, opening an interesting field for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
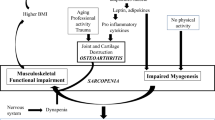
Musculoskeletal disorders (MSKD) affect bones, joints, ligaments, and muscles and are among the most common health problems in older people, affecting up to 80% of people over 65 [1]. MSKD in older adults are associated with disability [2], loss of independence, poor quality of life [3], mortality [4], and high healthcare costs [5]. The annual cost of MSKD is approximately 1 billion British pounds in the UK, and over 80 billion euros globally [5]. The most prevalent MSKD in older persons are osteoarthritis, osteoporosis (bone loss that predisposes to fractures), inflammatory arthritis (i.e., rheumatoid arthritis (RA)) and sarcopenia (loss of muscle mass, strength and/or function) [1]. Some MSKD can coexist in the same patient enhancing their harmful effects on health. Osteosarcopenia is a well-defined syndrome of concurrent osteoporosis and sarcopenia, contributing to adverse outcomes and reduced functional capacity [6, 7]. Over 144,000 osteoporotic fractures are reported yearly, while 40% of the ‘high-risk’ population suffering prior falls also presented with osteosarcopenia [8].
Currently, MSKD treatment involves medications and non-pharmacological (i.e., exercise and nutrition) interventions. However, available medications for osteoporosis and RA are limited by side effects, highly prevalent in older adults, and although non-pharmacological interventions, such as exercise, are effective in sarcopenia and osteoarthritis, they are affected by reduced adherence and baseline function [9]; thus, the search for novel therapeutic alternatives is mandatory.
The close relationship between MSKD and age suggests that common mechanisms between the biology of aging and the pathophysiology of MSKD may exist. In this sense, emerging anti-aging therapies could have a therapeutic role in managing MSKD. The mammalian target of rapamycin (mTOR) is a kinase that regulates several cellular aging processes, including cell growth, translation, and autophagy [10,11,12]. The inhibition of mTOR activity, by genetics or through pharmacological interventions, has increased maximal lifespan and health span in several animal species and is currently one of the most studied anti-aging interventions. Rapamycin, a natural macrocyclic lactone produced by the bacterium Streptomyces hygroscopicus, is a well-known mTOR inhibitor that can be administered in humans. Rapamycin binds to the immunophilin FK Binding Protein-12 (FKBP-12) in mammalian cells to generate a complex that binds to and inhibits mTOR activation [13]. Analogues of rapamycin (“rapalogues”) have been developed to optimize the pharmacokinetics of rapamycin-mediated mTOR inhibition leading to more favourable clinical outcomes [14, 15].
Preclinical data has demonstrated that rapamycin or rapalogue-induced mTOR inhibition protects against MSKD. Luo et al. [16] administered rapamycin to 24-month-old rats, resulting in increased trabecular bone mineralization associated with declining osteoclasts and elevated autophagy activity in osteocytes. Other studies in mice also show that the use of rapamycin or rapalogues ameliorates age-related muscle atrophy [17], whereas chronic mTOR activity leads to a decline in skeletal muscle mass [18]. These studies support targeting mTOR activity as a potential treatment for osteoporosis, sarcopenia, or osteosarcopenia. In other murine models of osteoarthritis, targeting mTOR activity with rapamycin delays or reduces joint cartilage degradation [19, 20]. In addition, rapamycin’s immunosuppressive effects have been used with clinical efficacy in cancer patients (e.g., renal cell carcinoma) [13], supporting rapamycin’s anti-inflammatory action and potential as a treatment for inflammatory MSKD (e.g., RA).
The consistency of improved outcomes following targeting mTOR activity across experimental models of MSKD calls for an investigation of the usefulness of rapamycin and rapalogues for treating chronic age-related MSKD in humans. Therefore, this systematic review aims to identify clinical studies using rapamycin and rapalogues to better understand their effects on the musculoskeletal system and address their potential therapeutic value.
Methods
Search strategy
This review was registered at PROSPERO (University of York) with registration number CRD42020208167 and conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). The search included MEDLINE, EMBASE, EMCARE, and the Cochrane Controlled Register of Trials. Results comprised papers available from September 2021 to the inception of the database. Key terms for interventions included the following: Rapamycin, rapalog*, Sirolimus, Everolimus, Temsirolimus, Ridaforolimus, Deforolimus and Zotarolimus. Terms used for conditions of interest included: muscle atrophy, sarcopenia, osteoporosis, bone disease, bone erosion, bone fragility, osteosarcopenia, demineralization, metabolic bone, osteoarthritis, and rheumatoid arthritis. The search also included terms that the MeSH dictionary may miss. EMBASE and EMCARE were limited to only articles and articles in press for relevant study types. An example of the search is shown in Table 1.
Inclusion and exclusion criteria
Inclusion criteria:
-
Rapamycin and rapalogues used as interventions could include:
-
Sirolimus (rapamycin)
-
Temsirolimus
-
Everolimus
-
Ridaforolimus (deforolimus)
-
Zotarolimus
-
-
Age-related MSKD in the study should relate to either:
-
Bone mineral disorders
-
Skeletal muscle loss
-
Osteoarthritis
-
RA
-
-
Randomized control trials (RCT) or controlled clinical trials (CCT) that compared the use of the rapalogue/rapamycin, either as a monotherapy, combination therapy, or adjuvant, to the current standard treatment or placebo for the condition of interest.
-
Other types of trials or study types (e.g., single-arm clinical trials or cross-sectional studies) should include the indicated interventions either as monotherapy, combination therapy, or adjuvant as part of the intervention/exposure and evaluate their effects on the conditions of interest.
-
The intervention outcomes on the condition of interest should have the processes of measurement explained. This may include (depending on the study) physician assessment, imaging, bone density scans, blood markers, quality of life, mobility, records of falls and/or fractures, changes in weight, adverse events, and muscle mass or function changes.
-
Participants aged 18 or over.
-
Study in English or translated to English.
-
No restriction on publication date.
Exclusion criteria:
-
Animal or in vitro studies.
-
Case reports or reviews.
-
Outcome measure inappropriate (e.g., lacking information on how it was measured, only a summary of outcomes with no supporting information).
Data extraction
Both COVIDENCE and Google Sheets recorded the outcomes of risk assessment and relevant study data of each paper. Tools used for assessing the risk of bias included the Cochrane RoB 2.0 [21] and ROBINS-I [22]. The assessments were initially conducted independently by Hong Lin and Anthony Lim. Both assessors then discussed the outcomes to reach a consensus.
Results
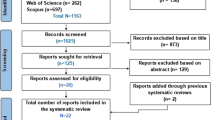
The literature search produced 850 results in total (Fig. 1). Six hundred and fifty results were retrieved for screening after removing duplicates (n = 200). After title/abstract screening and full-text review, fourteen studies were obtained for review. The main reasons for exclusion included: non-human study, participants under 18, inappropriate study protocol (incorrect or inconsistent study design), incorrect intervention, irrelevant outcomes, and non-English language. The search strategy flow chart is shown in Fig. 1
Risk of bias assessment
Risk of bias was assessed for all studies included in this review and is shown in Table 2.
Main outcomes
This systematic review aims to provide insights into the effect/s of rapamycin and rapalogues on the most prevalent age-related MSKD in human participants. Some studies measured multiple outcomes, so we focused on measurements/outcomes relevant to the review. Study characteristics are summarized in Table 3.
Bone changes
Five studies included the effects of rapamycin and rapalogues on bone as an outcome [23,24,25,26,27]. Campistol et al. [23] investigated the effects of rapamycin on bone metabolism in renal transplant patients, using serum osteocalcin and urinary N-telopeptide (i.e., bone-associate collagen degradation) as proxy markers of bone anabolism and catabolism. Reduced serum osteocalcin was observed in participants receiving rapamycin treatment than in those treated with the immunosuppressant cyclosporine A (P < 0.001 for weeks 12 and 24, and P < 0.008 at week 52). A significant reduction in urinary N-telopeptide levels in participants receiving rapamycin was also observed at week 24 (P = 0.018), and this remained consistently lower than in participants receiving cyclosporine A.
Westenfeld et al. [24] compared the effects of rapamycin vs. calcineurin inhibitor-based immunosuppression therapy in a cross-sectional study of renal transplant patients. The authors suggested that rapamycin could promote bone health by reducing osteoclast maturation and activity. They used serum TRAP-5b and RANKL as markers of bone metabolism and osteoclast differentiation. Compared to calcineurin inhibitor-based immunosuppression therapy, the study found significantly lower levels of TRAP-5b (P < 0.05; and P = 0.018 when accounting for confounders), and sRANKL (P < 0.05) in those treated with rapamycin. Complementary in vitro data showed rapamycin treatment suppressed osteoclast maturation, confirming that TRAP-5b was a more specific marker of osteoclast activity.
Sessa et al. [25] investigated the impact of various immunosuppressive regimens on different post-transplant renal osteopathy prevalence factors. Mean values of calcitonin (a thyroid hormone for calcium homeostasis) were higher in a group using tacrolimus combination therapy compared with the rapamycin combination group (P = 0.048). The results of this study did not otherwise show an effect on bone health that could be attributed to rapamycin. However, this study is conducted on a population of renal transplant patients who have likely had long-term osteodystrophy changes. As noted by the authors, treatment should start early as bone loss occurs just months post-transplantation [25].
Gnant et al. [26] examined the effects of everolimus with exemestane on bone marker levels in postmenopausal women with breast cancer. Overall, additional treatment with everolimus significantly decreased the serum bone markers bone-specific alkaline phosphatase (BSAP), procollagen type 1 N-terminal polypeptide (P1NP), and C-terminal cross-linking telopeptide type 1 collagen (CTX) from baseline, compared to the exemestane-only group (P < 0.01) at week 6. This trend generally continued onto the 12th week. Further, those with baseline bone metastases also had lower turnover markers than participants with no baseline bone metastases. The author attributed these findings to the additional anti-cancer effects of everolimus and the suppression of osteoclastogenesis.
Similarly, Hadji et al. [27] also investigated the effects of everolimus and exemestane therapy on bone in postmenopausal women with breast cancer [27]. However, patients were allowed prior/concomitant antiresorptive treatment (ART), and no controls were used. The authors’ conclusions were consistent with Gnant et al. [26] in that everolimus impaired osteoclast maturation and reduced bone turnover [26]. Significant changes were reported for all markers at 24 weeks [27], where mean changes from baseline for procollagen type I N-terminal propeptide (P1NP), osteocalcin, parathyroid hormone (PTH), 25-OH-vitamin D, and CTX were all reduced (P < 0.001) except for CTX (P = 0.036). ART patients also had a more significant reduction than those not receiving ART. Interestingly, the presence of baseline bone metastases did not meaningfully impact bone marker levels [27], opposing Gnant et al.’s [26] previous findings. This may be due to a few reasons: (1) a difference in the proportion of bone metastases between studies (59.3% in Hadji et al. [27] vs. 76.7% in Gnant et al. [26]), (2) a difference in the baseline bisphosphonate use (24.1% in Hadji et al. [27] vs. 47.2% in Gnant et al. [26]).
Skeletal muscle changes
Six studies examined the impact of rapalogues on skeletal muscle health and function as an outcome [28,29,30,31,32,33]. Gundermann et al. [28], Dickinson et al. [29, 30], and Drummond et al. [31] followed similar trial protocols.
Drummond et al. [31] demonstrated that rapamycin inhibited contraction-induced skeletal muscle protein synthesis (“SKMPS”) vs. the control (P < 0.05). Similarly, Gundermann et al. [28] showed that rapamycin inhibited SKMPS in the context of blood flow restriction exercise. Here, SKMPS was unchanged at all the time points for the rapamycin group (P < 0.05), whereas the control group demonstrated elevated levels of SKMPS (P < 0.05).
In one study, Dickinson et al. [29] observed that rapamycin inhibited L-essential amino acid (EAA) and stimulated SKMPS (P < 0.05). In a separate study, the same team [30] analyzed rapamycin’s effect on post-absorptive SKMPS or breakdown. Basal skeletal muscle protein metabolism changes were reported as insignificant following short-term administration of rapamycin (P > 0.05), concluding that rapamycin may only inhibit muscle synthesis in the presence of stimuli such as mechanical contractions or increased levels of EEAs.
Veasey-Rodrigues et al. [32] performed a pilot study analyzing body composition changes over 8 weeks from using Temsirolimus in patients with advanced solid tumours. Results show that there were no significant changes in body composition from baseline, such as skeletal muscle area (P = 0.57), skeletal muscle index (SMI) (P = 0.36) and lean body mass (LBM) (P = 0.56).
Gyawali et al. [33] conducted a retrospective study investigating the effect of long-term everolimus or temsirolimus use (> 6 months) in renal/pancreatic cancer patients. Long-term use of these rapalogues decreased skeletal muscle tissue (SMT) (P = 0.011), SMI (P = 0.022), and LBM (P = 0.007). However, changes in body weight were insignificant (P = 0.721). In this case, the explanation for the skeletal muscle loss was cachexia. However, the authors noted a 6-month use of rapalogues as an inclusion criterion, allowing the change in muscle mass to be reliably associated with the rapalogues instead of cancer cachexia. Interestingly, as their study population involved cancer patients, the following mechanisms of muscle wasting were relevant: elevated cytokines, reduced physical activity, and altered metabolism.
Rheumatoid arthritis
Three studies were identified [34,35,36], which examined the effects of rapamycin and rapalogues on RA. Bruyn et al. [34] investigated whether combinatorial treatment with everolimus and methotrexate could improve outcomes [34]. At 12 weeks, the everolimus and methotrexate combination group had a better response through the ACR20 (a criterion used to determine RA improvement) assessment (P = 0.022). The patient’s assessment of disease activity showed everolimus responded better (P = 0.004), and the clinical response compared to baseline was also significant (P = 0.024).
Wen et al. [35] studied the effects of low dose rapamycin on disease activity and immunological cells in patients with RA. Participants administered rapamycin were allowed to use other immunosuppressants. The results illustrated a clinical improvement through the decrease in the DAS28-ESR (an assessment of RA severity) score [37] from week 3 to week 24 (P < 0.001) from baseline. However, this decrease was insignificant compared to the control group on conventional treatment. The study acknowledged that participants on disease-modifying anti-rheumatic medications (DMARDs) had reduced the doses if given rapamycin. Moreover, the number of regulatory T cells (Treg) was higher compared to the conventional group (P < 0.05) at week 24. Wen et al. [35] provide insight into why anti-inflammatory Treg cells may improve RA in the long term.
Likewise, Niu et al. [36] explored the use of low dose rapamycin in low disease activity RA patients to determine its effect on Treg and other immune cells. The authors' deduction of why improvements in Treg levels can be attributed to the addition of rapamycin was consistent with Wen et al. [35]. At 12 weeks, Niu et al. [36] showed an increase in absolute count of the number of Treg cells in the rapamycin treatment group (P = 0.013) and percentage (P = 0.02) compared to baseline. Pro-inflammatory T-helper cell 17 (Th17) to anti-inflammatory Treg ratios were also significantly improved from baseline for those administered Rapamycin (P = 0.005), which was not the case in the conventional treatment group (P = 0.655). For the rapamycin treatment group, the clinical response was positive but not significant by the end of the study (DAS28 2.25 vs. 2.53 at the beginning). Furthermore, rapamycin treatment had a higher number of patients that were in DAS28 remission (DAS28 < 2.6) compared to baseline (71.4%). Those that achieved or remained in remission also had higher Treg levels.
The studies included in this review report adverse effects associated with using rapalogues in humans. A summary of the key adverse events and safety issues is listed in Table 4.
Discussion
To our knowledge, this is the first systematic review on the effect of rapamycin and rapalogues on MSKD in humans. Although these novel therapeutic approaches have been tested in humans and have shown beneficial effects on MSKD, mainly bone and joints, the evidence supporting their use in humans for these conditions is still limited.
Regarding bone health, most studies showed that rapamycin and rapalogues positively regulate bone turnover via a reduction in osteoclastogenesis [23, 24, 26, 27]. However, it is crucial to consider that the clinical context in which these interventions were evaluated does not correspond merely to aging. Among the studied populations, osteoporosis risk factors were highly prevalent: chronic steroid use, low oestrogen, low vitamin D, and immobilization [38], and many of these factors may contribute as confounding, such as renal osteodystrophy, post-transplant hyperparathyroidism, and increased bone resorption associated with aromatase inhibitor therapy and bone metastases [39, 40]. Finally, although the reduction in osteoclastogenesis is a promising outcome, these studies would ideally include more relevant clinical outcomes such as bone density by DXA scans [41] to quantify rapamycin’s effects on bone density.
In opposition to what was found in basic studies where an anabolic effect of rapalogues on muscle mass was observed, three studies [29,30,31] showed negative effects of rapamycin and rapalogues on skeletal muscle metabolism. However, it is important to analyze the population studied and the protocol with which the intervention was implemented. The participants in these studies were young, healthy participants, and the intervention was short in time. As mTORC1 activity increases in aging and contributes to muscle loss [18], extrapolating the short-term effects of rapamycin to long-term outcomes of muscle maintenance/loss is too speculative. Furthermore, skeletal muscle composition differs significantly in aged individuals due to long term changes in muscle mass, reduced hormone synthesis (e.g., growth hormone, oestrogen), the presence of inflammatory cytokines and adipokines, and decreased physical activity [6]. While our review aims to comment on rapamycin and rapalogues for use in an aged population, it is still informative to observe how rapamycin and rapalogues affect healthy muscle as a rationale for future clinical trials in older participants. Longer-term studies of rapamycin treatment included cancer patients [32, 33]. This introduces different muscle wasting pathologies: MSKD and disuse, vs. chronic disease and muscle atrophy, or both [42, 43]. One study proposed muscle loss from long-term rapamycin use was likely due to chronic mTORC1 inhibition [33]; however, the lack of a control group and short follow-up time limit these findings. Notably, these studies [32, 33] involved participants with median ages more representative of an aging population, i.e., when age-related muscle pathology and the gradual nature of sarcopenia can manifest [44].
In RA, rapamycin improved biochemical and clinical outcomes of patients receiving methotrexate while having comparable side effects [34]. This may be due to both rapamycin and methotrexate targeting cell proliferation pathways. This is evidenced by Wen et al. [35], who observed decreased DMARD intake when rapamycin was added to the therapeutic regimen. Future studies should confirm the safety and efficacy of combinatorial rapamycin (or rapalogues) and DMARD therapies.
The toxicity profile of rapamycin is different in humans than in animal models and is an important point to discuss. Common adverse reactions in humans include immunosuppression, oral ulcers hyperglycaemia/diabetes, hyperlipidaemia and hypercholesterolemia [12]. Mannick et al. [45] reported on everolimus and concluded it was safe among subjects over 65 years. Similarly, Kraig et al. [46] reported that 8 weeks of rapamycin was safely tolerated by subjects 70–95 years otherwise healthy. However, there were trends for increased HbA1c and cholesterol [46] and similar views on side effects were shared in the studies included in this systematic review. Short-term rapamycin administration and/or low doses of the drug did not produce any major concerns over side effects [28,29,30,31, 35, 36]. However, longer-term studies showed increased metabolic disturbances such as hyperlipidaemia and hyperglycaemia [23, 24, 26, 27, 32, 34]. These metabolic consequences [47, 48] may be an issue in patients treated with rapamycin, who also present with cardiovascular disease (or risk factors thereof). Overall, while rapamycin is generally safe, further studies are required to ensure common side effects can be safely managed. Future studies should also determine the “optimal dose” of rapamycin and rapalogues that produce the desired effects.
Limitations of this systematic review include the low number of studies and the diverse study designs. There are differing characteristics between study participants (e.g., young age) and the population of interest (e.g., aging population). This systematic review aimed to broadly appraise current literature involving human participants by having inclusion criteria encompassing multiple study types, meaning different levels of evidence were included, and exposure to other biases was possible. Such study types comprise non-randomized trials, non-blinded studies, cross-sectional studies, cohort studies, single-arm trials, and secondary analyses of other trials. Other issues with some study designs would be the smaller sample sizes, different dosages of the drug, co-interventions varying between groups and shorter follow-up duration.
Conclusion
This is the first study looking at the effect of rapamycin and rapalogues on MSKD. Studies included in this review have mostly evaluated the effect of rapamycin and rapalogues on MSKD in humans, showing anabolic effects on bone metabolism and reducing the inflammatory activity of RA. However, the evidence supporting its use is still incipient, and the clinical implication of these results on the management of osteoporosis, sarcopenia, or osteosarcopenia has not been studied, opening an interesting field for future research.
Availability of data and materials
Data available on request from the authors.
Code availability
Not applicable.
References
Smith TO, Purdy R, Latham SK et al (2016) The prevalence, impact and management of musculoskeletal disorders in older people living in care homes: a systematic review. Rheumatol Int 36:55–64
Brooks PM (2006) The burden of musculoskeletal disease–a global perspective. Clin Rheumatol. 5:778–781
Forjaz M, Rodriguez-Blazquez C, Ayala A et al (2015) Chronic conditions, disability, and quality of life in older adults with multimorbidity in Spain. Eur J Intern Med 26:176–181
Salech F, Marquez C, Lera L et al (2021) Osteosarcopenia predicts falls, fractures, and mortality in chilean community-dwelling older adults. J Am Med Dir Assoc 22:853–858
Lewis R, Gómez Álvarez CB, Rayman M et al (2019) Strategies for optimizing musculoskeletal health in the 21st century. BMC Musculoskelet Disord 20:164
Kirk B, Zanker J, Duque G (2020) Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle 11:609–618
Paintin J, Cooper C, Dennison E (2018) Osteosarcopenia. Br J Hosp Med (Lond) 79:253–258
Hassan E, Duque G (2017) Osteosarcopenia: a new geriatric syndrome. Aust Fam Phys 46:849–853
Law TD, Clark LA, Clark BC (2016) Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu Rev Gerontol Geriatr 36:205–228
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168:960–976
Kennedy BK, Berger SL, Brunet A et al (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713
Selvarani R, Mohammed S, Richardson A (2020) Effect of Rapamycin on aging and age-related diseases—past and future. GeroScience 43:1135–1158
Waldner M, Fantus D, Solari M et al (2016) New perspectives on mTOR inhibitors (Rapamycin, rapalogs and TORKinibs) in transplantation. Br J Clin Pharmacol 82:1158–1170
Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA et al (2019) A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun 10:3194
Abdel-Magid AF (2019) Rapalogs potential as practical alternatives to rapamycin. ACS Med Chem Lett 10:843–845
Luo D, Ren H, Li T et al (2016) Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int 27:1093–1101
Tang H, Inoki K, Brooks SV et al (2019) mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 18:e12943
Yoon MS (2017) mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 8:788
Takayama K, Kawakami Y, Kobayashi M, Greco N et al (2014) Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther 16:482
Caramés B, Hasegawa A, Taniguchi N, Miyaki S et al (2012) Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis 71:575–581
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Campistol JM, Holt DW, Epstein S et al (2005) Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl Int 18:1028–1035
Westenfeld R, Schlieper G, Wöltje M et al (2011) Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis—implications for post-transplantation bone disease. Nephrol Dial Transp 26:4115–4123
Sessa A, Esposito A, Iavicoli GD et al (2010) Immunosuppressive agents and bone disease in renal transplant patients with hypercalcemia. Transplant Proc 42:1148–1155
Gnant M, Baselga J, Rugo HS et al (2013) Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. JNCI 105:654–63
Hadji P, Stoetzer O, Decker T et al (2019) The impact of mammalian target of rapamycin inhibition on bone health in postmenopausal women with hormone receptor-positive advanced breast cancer receiving everolimus plus exemestane in the phase IIIb 4EVER trial. J Bone Oncol 14:010–10
Gundermann DM, Walker DK, Reidy PT et al (2014) Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 306:E1198–E1204
Dickinson JM, Fry CS, Drummond MJ et al (2011) Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141:856–862
Dickinson JM, Drummond MJ, Fry CS et al (2013) rapamycin does not affect post-absorptive protein metabolism in human skeletal muscle. Metabolism 62:144–151
Drummond MJ, Fry CS, Glynn EL et al (2009) Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587:1535–1546
Veasey-Rodrigues H, Parsons HA, Janku F et al (2013) A pilot study of Temsirolimus and body composition. J Cachexia Sarcopenia Muscle 4:259–265
Gyawali B, Shimokata T, Honda K et al (2016) Muscle wasting associated with the long-term use of mTOR inhibitors. Mol Clin Oncol 5:641–646
Bruyn GA, Tate G, Caeiro F et al (2008) Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomized, placebo-controlled, parallel-group, proof-of-concept study. Ann Rheum Dis 67:1090–1095
Wen HY, Wang J, Zhang SX et al (2019) Low-dose sirolimus immunoregulation therapy in patients with active rheumatoid arthritis: a 24-week follow-up of the randomized, open-label, parallel-controlled trial. J Immunol Res 2019:7684352
Niu HQ, Li ZH, Zhao WP et al (2020) Sirolimus selectively increases circulating Treg cell numbers and restores the Th17/Treg balance in rheumatoid arthritis patients with low disease activity or in DAS28 remission who previously received conventional disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol 38:58–66
The DAS28 score (2020) Nation Rheumatoid Arthritis Society. https://nras.org.uk/resource/the-das28-score. Accessed Oct 2020
Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. RACGP. 2020. https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis. Accessed Oct 2020.
Maalouf NM, Shane E (2005) Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab 90:2456–2465
Perez EA, Weilbaecher K (2006) Aromatase inhibitors and bone loss. Oncology (Williston Park) 20:1029–1048
Phillips PJ, Phillipov G (2006) Bone mineral density—frequently asked questions. Aust Fam Phys 35:341–344
Ali S, Garcia JM (2014) Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options—a mini-review. Gerontology 60:294–305
Barreiro E, Jaitovich A (2018) Muscle atrophy in chronic obstructive pulmonary disease: molecular basis and potential therapeutic targets. J Thorac Dis 10:S1415–S1424
Kim TN, Choi KM (2013) Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab 20:1–10
Mannick JB, Del Giudice G, Lattanzi M et al (2014) mTOR inhibition improves immune function in the elderly. Sci Transl Med 6:268ra179
Kraig E, Linehan LA, Liang H et al (2018) A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol 105:53–69
Hyperglycemia in diabetes—symptoms and causes. Mayo Clinic. 2021. https://www.mayoclinic.org/diseases-conditions/hyperglycemia/symptoms-causes/syc-20373631. Accessed Mar 2021
Heart disease—know your risk. BetterHealth Channel. 2021. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/heart-disease-risk-factors#diabetes-and-cardiovascular-disease-risk. Accessed Mar 2021
Eghtesad S, Jhunjhunwala S, Little SR et al (2011) Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Mol Med 17:917–924
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This review was funded via a training grant from the Australian Institute for Musculoskeletal Science (AIMSS).
Author information
Authors and Affiliations
Contributions
GD conceived the initial idea for the review, edited the manuscript, and supervised Hong Lin during the review. HL conducted the search, analyzed data, and wrote the initial draft for the manuscript. FS reviewed and edited the manuscript. AL assisted with data screening, data extraction, editing, and referencing. SV provided statistical and risk assessment advice.
Corresponding author
Ethics declarations
Conflicts of interest
None declared.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, H., Salech, F., Lim, A. et al. The effect of rapamycin and its analogues on age-related musculoskeletal diseases: a systematic review. Aging Clin Exp Res 34, 2317–2333 (2022). https://doi.org/10.1007/s40520-022-02190-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02190-0