Abstract
Background
Previous studies have evaluated the prognostic effects of sarcopenia in cancer patients receiving various treatments, including chemotherapy and surgery, but few studies have focused on radiotherapy (RT).
Aims
We aimed to investigate the prevalence of sarcopenia and the relationship between sarcopenia and outcomes in older cancer patients who underwent RT without chemotherapy.
Methods
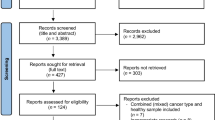
A systematic review of the literature was conducted in Pubmed/Medline and Cochrane databases in September 2021. We used the search terms and medical subject heading terms “sarcopenia,” “low muscle mass (LMM),” “low muscle strength,” “LMM and low muscle strength,” “LMM and low muscle strength and low physical performance,” and “RT.” Outcomes were overall survival (OS), progression-free survival, non-cancer death, cancer death, disease-specific survival, local failure-free survival, distant failure-free survival, and RT-related toxicities.
Results
Among 460 studies, 8 studies were eligible for inclusion. The prevalence of sarcopenia was between 42.8% and 72%. Sarcopenia was not associated with OS or OS at 3 years in seven studies in which it was defined as the presence of LMM, while it was related in one study, in which it was defined as the concomitant presence of LMM and muscle strength/function.
Discussion
There was heterogeneity between the studies because there was diversity in their inclusion criteria, definition and assessment methods used for detection of sarcopenia, considered cutoffs for low muscle mass and strength, cross-sectional locations on imaging to assess muscle mass and included covariates. The discrepancy in the results of the studies may also result from the variations in diagnoses, sample sizes, and treatment modalities. The low number of included studies and a small number of patients in each study limited generalizability.
Conclusions
Sarcopenia may be a prognostic factor, especially in OS when low muscle strength/function is integrated into its definition. We suggest that clinicians focus on muscle strength/function while considering sarcopenia and its association with cancer and RT-related outcomes.

Similar content being viewed by others
Availiability of data
The data is available from the authors upon reasonable request.
References
World Population Ageing Report (2015) United Nations, Department of Economic and Social Affairs, Population Division (ST/ESA/SER.A/390)
Bahat G, Erdogan T. Sarkopeni. Geriatri, Yaşlı Sağlığı ve Hastalıkları Kitabı. Erdincler UDS, Karan MA, Ulger Z (Editors). (2021)
Hernandez Torres C, Hsu T (2017) Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus 3:330–339. https://doi.org/10.1016/j.euf.2017.10.010
Colloca G, Tagliaferri L, Capua BD et al (2020) Management of the elderly cancer patients complexity the radiation oncology potential. Aging Dis 11:649–657. https://doi.org/10.14336/AD.2019.0616
Delaney G, Jacob S, Featherstone C et al (2005) The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104:1129–1137. https://doi.org/10.1002/cncr.21324.Erratum.In:Cancer.2006;107(3):660
Popescu T, Karlsson U, Vinh-Hung V et al (2019) Challenges facing radiation oncologists in the management of older cancer patients: consensus of the international geriatric radiotherapy group. Cancers (Basel) 11:371. https://doi.org/10.3390/cancers11030371
Sis Çelik A (2014) Radyoterapi Sonucu Gelişen Yan Etkiler ve Hemşirelik Yaklaşımı. Gümüşhane Üniversitesi Sağlık Bilimleri Dergisi/Gümüşhane University. J Health Sci 3(3):933–947. https://dergipark.org.tr/tr/pub/gumussagbil/issue/23832/253887
Wang S, Liu Y, Feng Y et al (2019) A review on curability of cancers: more efforts for novel therapeutic options are needed. Cancers (Basel) 11:1782. https://doi.org/10.3390/cancers11111782
Rim CH, Yoon WS, Lee JA et al (2018) Factors predicting intolerance to definitive conventional radiotherapy in geriatric patients. Strahlenther Onkol 194:894–903. https://doi.org/10.1007/s00066-018-1318-y
Rieger KE, Hong WJ, Tusher VG et al (2004) Toxicity from radiation therapy associated with abnormal transcriptional responses to DNA damage. Proc Natl Acad Sci U S A 101:6635–6640. https://doi.org/10.1073/pnas.0307761101
Shachar SS, Williams GR, Muss HB et al (2016) Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer 57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030
Anjanappa M, Corden M, Green A et al (2020) Sarcopenia in cancer: Risking more than muscle loss. Tech Innov Patient Support Radiat Oncol 16:50–57. https://doi.org/10.1016/j.tipsro.2020.10.001
Taylor JM, Song A, David AR et al (2020) Impact of sarcopenia on survival in patients with early-stage lung cancer treated with stereotactic body radiation therapy. Cureus 12:e10712. https://doi.org/10.7759/cureus.10712
Hua X, Liu S, Liao JF et al (2020) When the loss costs too much: a systematic review and meta-analysis of sarcopenia in head and neck cancer. Front Oncol 9:1561. https://doi.org/10.3389/fonc.2019.01561
Dunne RF, Loh KP, Williams GR et al (2019) Cachexia and sarcopenia in older adults with cancer: a comprehensive review. Cancers (Basel) 11:1861. https://doi.org/10.3390/cancers11121861
Vangelov B, Bauer J, Kotevski D et al (2021) The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: a systematic review. Br J Nutr. https://doi.org/10.1017/S0007114521001446
Fintelmann FJ, Troschel FM, Mario J et al (2018) Thoracic skeletal muscle is associated with adverse outcomes after lobectomy for lung cancer. Ann Thorac Surg 105:1507–1515. https://doi.org/10.1016/j.athoracsur.2018.01.013
Madariaga MLL, Troschel FM, Best TD et al (2020) Low thoracic skeletal muscle area predicts morbidity after pneumonectomy for lung cancer. Ann Thorac Surg 109:907–913. https://doi.org/10.1016/j.athoracsur.2019.10.041
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
Wells G, Shea B, O'Connell D et al (2014) The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Pielkenrood BJ, van Urk PR, Velden JM et al (2020) Impact of body fat distribution and sarcopenia on the overall survival in patients with spinal metastases receiving radiotherapy treatment: a prospective cohort study. Acta Oncol 59:291–297. https://doi.org/10.1080/0284186X.2019.1693059
Matsuo Y, Nagata Y, Wakabayashi M et al (2021) Impact of pre-treatment C-reactive protein level and skeletal muscle mass on outcomes after stereotactic body radiotherapy for T1N0M0 non-small cell lung cancer: a supplementary analysis of the Japan Clinical Oncology Group study JCOG0403. J Radiat Res 62:901–909. https://doi.org/10.1093/jrr/rrab065
Kabarriti R, Bontempo A, Romano M et al (2018) The impact of dietary regimen compliance on outcomes for HNSCC patients treated with radiation therapy. Support Care Cancer 26:3307–3313. https://doi.org/10.1007/s00520-018-4198-x
Chargi N, Bril SI, Emmelot-Vonk MH et al (2019) Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol 276:1475–1486. https://doi.org/10.1007/s00405-019-05361-4
Shiba S, Shibuya K, Katoh H et al (2018) No Deterioration in Clinical Outcomes of Carbon Ion Radiotherapy for Sarcopenia Patients with Hepatocellular Carcinoma. Anticancer Res 38:3579–3586. https://doi.org/10.21873/anticanres.12631
Matsuo Y, Mitsuyoshi T, Shintani T et al (2018) Impact of low skeletal muscle mass on non-lung cancer mortality after stereotactic body radiotherapy for patients with stage I non-small cell lung cancer. J Geriatr Oncol 9:589–593. https://doi.org/10.1016/j.jgo.2018.05.003
Stangl-Kremser J, D’Andrea D, Vartolomei M et al (2019) Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol Oncol 37:372–379. https://doi.org/10.1016/j.urolonc.2018.11.001
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926. https://doi.org/10.1158/1078-0432.CCR-08-2242
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635. https://doi.org/10.1016/S1470-2045(08)70153-0
Harimoto N, Shirabe K, Yamashita YI et al (2013) Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 100:1523–1530. https://doi.org/10.1002/bjs.9258
Parkin E, Plumb AA, O'Reilly D et al (2012) Body composition and outcome in patients undergoing resection of colorectal liver metastases (Br J Surg 2012; 99: 550–557). Br J Surg. 2012;99(7):1021–2; author reply 1022. https://doi.org/10.1002/bjs.8826
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Wendrich AW, Swartz JE, Bril SI et al (2017) Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol 71:26–33. https://doi.org/10.1016/j.oraloncology.2017.05.012
Jones KI, Doleman B, Scott S et al (2015) Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 17:O20–O26. https://doi.org/10.1111/codi.12805
Gu DH, Kim MY, Seo YS et al (2018) Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol 24:319–330. https://doi.org/10.3350/cmh.2017.0077
Nagata Y, Hiraoka M, Shibata T et al (2015) Prospective trial of stereotactic body radiation therapy for both operable and inoperable t1n0m0 non-small cell lung cancer: japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys 93:989–996. https://doi.org/10.1016/j.ijrobp.2015.07.2278
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S-991S. https://doi.org/10.1093/jn/127.5.990S
Bahat G, Tufan A, Tufan F et al (2016) Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 35:1557–1563. https://doi.org/10.1016/j.clnu.2016.02.002
Bahat G, Ihan B (2016) Sarcopenia and the cardiometabolic syndrome: A narrative review. European Geriatric Medicine. https://doi.org/10.1016/j.eurger.2015.12.012
Arango-Lopera VE, Arroyo P, Gutiérrez-Robledo LM et al (2013) Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging 17:259–262. https://doi.org/10.1007/s12603-012-0434-0
Sobestiansky S, Michaelsson K, Cederholm T (2019) Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr 19:318. https://doi.org/10.1186/s12877-019-1338-1
Wang H, Hai S, Liu Y et al (2019) Skeletal Muscle Mass as a Mortality Predictor among Nonagenarians and Centenarians: A Prospective Cohort Study. Sci Rep 9:2420. https://doi.org/10.1038/s41598-019-38893-0
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520.Erratum.In:AmJEpidemiol1999;149(12):1161
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(4):601. https://doi.org/10.1093/ageing/afz046. Erratum for: Age Ageing. 2019;48(1):16–31
Bahat G, Tufan A, Kilic C et al (2019) Cut-off points for weight and body mass index adjusted bioimpedance analysis measurements of muscle mass. Aging Clin Exp Res 31:935–942. https://doi.org/10.1007/s40520-018-1042-6
Bahat G, Turkmen BO, Aliyev S et al (2021) Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr S0261–5614:00020. https://doi.org/10.1016/j.clnu.2021.01.010
Bahat G, Tufan A, Kilic C et al (2020) Cut-off points for height, weight and body mass index adjusted bioimpedance analysis measurements of muscle mass with use of different threshold definitions. Aging Male 23:382–387. https://doi.org/10.1080/13685538.2018.1499081
Li R, Xia J, Zhang XI et al (2018) Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 50:458–467. https://doi.org/10.1249/MSS.0000000000001448
Han P, Chen X, Yu X et al (2020) The predictive value of sarcopenia and its individual criteria for cardiovascular and all-cause mortality in suburb-dwelling older chinese. J Nutr Health Aging 24:765–771. https://doi.org/10.1007/s12603-020-1390-8
Williams GR, Chen Y, Kenzik KM et al (2020) Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Netw Open 3:e204783. https://doi.org/10.1001/jamanetworkopen.2020.4783
Bahat G, Tufan A, Kilic C et al (2018) Prevalence of sarcopenia and its components in community-dwelling outpatient older adults and their relation with functionality. Aging Male. https://doi.org/10.1080/13685538.2018.1511976
Fielding RA, Vellas B, Evans WJ et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 12:249–256. https://doi.org/10.1016/j.jamda.2011.01.003
Cederholm T, Barazzoni R, Austin P et al (2017) ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 36:49–64. https://doi.org/10.1016/j.clnu.2016.09.004
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69:547–558. https://doi.org/10.1093/gerona/glu010
Pamoukdjian F, Bouillet T, Lévy V et al (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr 37:1101–1113. https://doi.org/10.1016/j.clnu.2017.07.010
Fujiwara N, Nakagawa H, Kudo Y et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63:131–140. https://doi.org/10.1016/j.jhep.2015.02.031
Yip C, Goh V, Davies A et al (2014) Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 24:998–1005. https://doi.org/10.1007/s00330-014-3110-4
Matsuoka H, Nakamura K, Matsubara Y et al (2019) Sarcopenia Is Not a Prognostic Factor of Outcome in Patients With Cervical Cancer Undergoing Concurrent Chemoradiotherapy or Radiotherapy. Anticancer Res. 39:933–939. https://doi.org/10.21873/anticanres.13196
Kilgour RD, Vigano A, Trutschnigg B et al (2013) Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 21:3261–3270. https://doi.org/10.1007/s00520-013-1894-4
Newman AB, Kupelian V, Visser M et al (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61:72–77. https://doi.org/10.1093/gerona/61.1.72
Bahat G, Kilic C, Altinkaynak M et al (2020) Comparison of standard versus population-specific handgrip strength cut-off points in the detection of probable sarcopenia after launch of EWGSOP2. Aging Male 23:1564–1569. https://doi.org/10.1080/13685538.2020.1870038
Findlay M, White K, Lai M et al (2020) The Association Between Computed Tomography-Defined Sarcopenia and Outcomes in Adult Patients Undergoing Radiotherapy of Curative Intent for Head and Neck Cancer: A Systematic Review. J Acad Nutr Diet 120:1330-1347.e8. https://doi.org/10.1016/j.jand.2020.03.021
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 39:412–423. https://doi.org/10.1093/ageing/afq034
Duan K, Gao X, Zhu D (2021) The clinical relevance and mechanism of skeletal muscle wasting. Clin Nutr 40:27–37. https://doi.org/10.1016/j.clnu.2020.07.029
Hamaguchi Y, Kaido T, Okumura S et al (2017) Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 101:565–574. https://doi.org/10.1097/TP.0000000000001587
Okumura S, Kaido T, Hamaguchi Y et al (2016) Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery 159:821–833. https://doi.org/10.1016/j.surg.2015.08.047
Bahat G, Catikkas NM, Karan MA (2021) Skeletal muscle mass assessment to detect low muscle mass: regional or total? Clin Nutr. https://doi.org/10.1016/j.clnu.2021.06.014
Van der Werf A, Langius JAE, de van der Schueren MAE et al (2018) Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 72:288–296. https://doi.org/10.1038/s41430-017-0034-5
Derstine BA, Holcombe SA, Ross BE et al (2018) Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8:11369. https://doi.org/10.1038/s41598-018-29825-5
Hamaguchi Y, Kaido T, Okumura S et al (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205. https://doi.org/10.1016/j.nut.2016.04.003
Kim JS, Kim WY, Park HK et al (2017) Simple age specific cutoff value for sarcopenia evaluated by computed tomography. Ann Nutr Metab 71:157–163. https://doi.org/10.1159/000480407
Ufuk F, Herek D (2019) Reference skeletal muscle mass values at L3 Vertebrae level based on computed tomography in healthy Turkish adults. Int J Gerontol 13:221e5
Hamaguchi Y, Kaido T, Okumura S et al (2018) Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J Cachexia Sarcopenia Muscle 9:246–254. https://doi.org/10.1002/jcsm.12276
Morishita S (2016) Prevalence of Sarcopenia in Cancer Patients: Review and Future Directions. International Journal of Physical Medicine & Rehabilitation. https://doi.org/10.4172/2329-9096.1000342
Ganju RG, Morse R, Hoover A et al (2019) The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol 137:117–124. https://doi.org/10.1016/j.radonc.2019.04.023
Morishita S, Kaida K, Tanaka T et al (2012) Prevalence of sarcopenia and relevance of body composition, physiological function, fatigue, and health-related quality of life in patients before allogeneic hematopoietic stem cell transplantation. Support Care Cancer 20:3161–3168. https://doi.org/10.1007/s00520-012-1460-5
Beaudart C, Zaaria M, Pasleau F et al (2017) Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 12:e0169548. https://doi.org/10.1371/journal.pone.0169548
Huillard O, Mir O, Peyromaure M et al (2013) Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer 108:1034–1041. https://doi.org/10.1038/bjc.2013.58
Bruyère O, Beaudart C, Ethgen O et al (2019) The health economics burden of sarcopenia: a systematic review. Maturitas 119:61–69. https://doi.org/10.1016/j.maturitas.2018.11.003
Acknowledgements
The authors certify that they comply with ethical guidelines for authorship and publishing of the Aging Clinical and Experimental Research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NMC: conceptualization, methodology, writing—original draft preparation, writing—review and editing. ZB: writing—original draft preparation, data curation, review and editing. MMO: formal analysis, data curation. GB: conceptualization, methodology, writing—original draft preparation, writing—review a editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Catikkas, N.M., Bahat, Z., Oren, M.M. et al. Older cancer patients receiving radiotherapy: a systematic review for the role of sarcopenia in treatment outcomes. Aging Clin Exp Res 34, 1747–1759 (2022). https://doi.org/10.1007/s40520-022-02085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02085-0