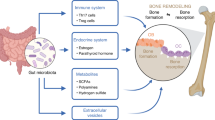
Abstract
The skeleton is the framework and in charge of body configuration preservation. As a living tissue, bones are constantly being formed and absorbed. Osteoblasts and osteoclasts are the main bone cells and balance between their activities indicates bone health. Several mechanisms influence the bone turnover and RANKL/RANK/OPG pathway is one of them. This system, whose components are part of the tumor necrosis factor (TNF) superfamily, exists in many organs and could play a role in bone modeling and remodeling. RANKL/RANK pathway controls osteoclasts activity and formation. In addition, they are identified as key factors on bone turnover in different pathological situations. At the same time, OPG (RANKL’s decoy receptor) plays role as a bone-protective factor by binding to RANKL and prevention of extra resorption. The lack of balance between RANKL and OPG could result in excessive bone resorption. Probiotics, the beneficial microorganisms for human health, entail bones in their advantages. Recent studies suggest that probiotics could reduce inflammatory factors (for example TNF-α and IL-1β) and increase bone OPG expression. In addition, probiotics have shown to maintain bones in various ways. Although current evidence is not enough for definitive approval of probiotics’ efficacy on RANKL/RANK/OPG, its positive responses from conducted studies are significant. Understanding of the probiotics’ effects on RANKL/RANK/OPG pathway will help focus future studies, and assist in developing efficient treatment strategies.


Similar content being viewed by others
References
Rizzoli R, Abraham C, Brandi ML (2014) Nutrition and bone health: turning knowledge and beliefs into healthy behaviour. Curr Med Res Opin 30:131–141. https://doi.org/10.1185/03007995.2013.847410
Office of the Surgeon G (2004) Reports of the surgeon general. In: Bone health and osteoporosis: a report of the surgeon general. Office of the Surgeon General (US), Rockville
Davidge Pitts CJ, Kearns AE (2011) Update on medications with adverse skeletal effects. Mayo Clin Proc 86:338–343. https://doi.org/10.4065/mcp.2010.0636 (quiz 343)
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Kennel KA, Drake MT (2009) Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 84:632–637. https://doi.org/10.1016/s0025-6196(11)60752-0 (quiz 638)
FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. WHO, Geneva
Sanders ME (2008) Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46(Suppl 2):S58–S61. https://doi.org/10.1086/523341 (discussion S144-151)
Fijan S (2014) Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 11:4745–4767. https://doi.org/10.3390/ijerph110504745
Sharon G, Garg N, Debelius J et al (2014) Specialized metabolites from the microbiome in health and disease. Cell Metab 20:719–730. https://doi.org/10.1016/j.cmet.2014.10.016
Singh N, Gurav A, Sivaprakasam S et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139. https://doi.org/10.1016/j.immuni.2013.12.007
Matsumoto S, Hara T, Hori T et al (2005) Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol 140:417–426
Lin YP, Thibodeaux CH, Peña JA et al (2008) Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14:1068–1083
Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S et al (2012) Probiotic mechanisms of action. Ann Nutr Metab 61:160–174. https://doi.org/10.1159/000342079
Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407. https://doi.org/10.1038/ncpgasthep0528
Gomez-Llorente C, Munoz S, Gil A (2010) Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 69:381–389. https://doi.org/10.1017/s0029665110001527
Abu-Amer Y (2013) NF-kappaB signaling and bone resorption. Osteoporos Int 24:2377–2386. https://doi.org/10.1007/s00198-013-2313-x
McCabe L, Britton RA, Parameswaran N (2015) Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep 13:363–371. https://doi.org/10.1007/s11914-015-0292-x
Ohlsson C, Sjogren K (2018) Osteomicrobiology: a new cross-disciplinary research field. Calcif Tissue Int 102:426–432. https://doi.org/10.1007/s00223-017-0336-6
Walsh MC, Takegahara N, Kim H et al (2018) Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat Rev Rheumatol 14:146–156. https://doi.org/10.1038/nrrheum.2017.213
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146. https://doi.org/10.1016/j.abb.2008.03.018
Tanaka Y, Nakayamada S, Okada Y (2005) Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy 4:325–328
Boyce BF, Xing L (2007) The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5:98–104
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055. https://doi.org/10.1210/endo.142.12.8536
Bruhn-Olszewska B, Korzon-Burakowska A, Wegrzyn G et al (2017) Prevalence of polymorphisms in OPG, RANKL and RANK as potential markers for Charcot arthropathy development. Sci Rep 7:501. https://doi.org/10.1038/s41598-017-00563-4
Wu PF, Tang JY, Li KH (2015) RANK pathway in giant cell tumor of bone: pathogenesis and therapeutic aspects. Tumour Biol 36:495–501. https://doi.org/10.1007/s13277-015-3094-y
Liu W, Zhang X (2015) Receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol Med Rep 11:3212–3218. https://doi.org/10.3892/mmr.2015.3152
Fan F, Shi P, Liu M et al (2018) Lactoferrin preserves bone homeostasis by regulating the RANKL/RANK/OPG pathway of osteoimmunology. Food Funct 9:2653–2660. https://doi.org/10.1039/c8fo00303c
Nelson CA, Warren JT, Wang MW et al (2012) RANKL employs distinct binding modes to engage RANK and the osteoprotegerin decoy receptor. Structure 20:1971–1982. https://doi.org/10.1016/j.str.2012.08.030
Ando K, Mori K, Redini F et al (2008) RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol 5:263–268
Jones RM, Mulle JG, Pacifici R (2018) Osteomicrobiology: the influence of gut microbiota on bone in health and disease. Bone 115:59–67. https://doi.org/10.1016/j.bone.2017.04.009
Sobel V, Schwartz B, Zhu Y-S et al (2006) Bone mineral density in the complete androgen insensitivity and 5α-reductase-2 deficiency syndromes. J Clin Endocrinol Metabol 91:3017–3023
McClung M (2007) Role of RANKL inhibition in osteoporosis. Arthritis Res Therapy 9(Suppl 1):S3. https://doi.org/10.1186/ar2167
Geusens P (2012) The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis 4:225–233. https://doi.org/10.1177/1759720x12438080
Walsh MC, Choi Y (2014) Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol 5:511. https://doi.org/10.3389/fimmu.2014.00511
Rizzoli R, Body JJ, Brandi ML et al (2013) Cancer-associated bone disease. Osteoporos Int 24:2929–2953. https://doi.org/10.1007/s00198-013-2530-3
Eslick R, Talaulikar D (2013) Multiple myeloma: from diagnosis to treatment. Aust Fam Physician 42:684–688
Schmiedel BJ, Scheible CA, Nuebling T et al (2013) RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Can Res 73:683–694. https://doi.org/10.1158/0008-5472.can-12-2280
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4:268–275
Renn TY, Huang YK, Feng SW et al (2018) Prophylactic supplement with melatonin successfully suppresses the pathogenesis of periodontitis through normalizing RANKL/OPG ratio and depressing the TLR4/MyD88 signaling pathway. J Pineal Res. https://doi.org/10.1111/jpi.12464
Weitzmann MN (2017) Bone and the immune system. Toxicol Pathol 45:911–924. https://doi.org/10.1177/0192623317735316
Nagy V, Penninger JM (2015) The RANKL-RANK Story. Gerontology 61:534–542. https://doi.org/10.1159/000371845
Ma YL, Cain RL, Halladay DL et al (2001) Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 14:4047–4054. https://doi.org/10.1210/endo.142.9.8356
Takegami N, Akeda K, Yamada J et al (2017) RANK/RANKL/OPG system in the intervertebral disc. Arthritis Res Therapy 19:121. https://doi.org/10.1186/s13075-017-1332-y
Ozaki Y, Koide M, Furuya Y et al (2017) Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis. PLoS ONE 12:e0184904. https://doi.org/10.1371/journal.pone.0184904
Gatto F, Redaelli D, Salvade A et al (2012) Hurler disease bone marrow stromal cells exhibit altered ability to support osteoclast formation. Stem Cells Dev 21:1466–1477. https://doi.org/10.1089/scd.2011.0555
Britton RA, Irwin R, Quach D et al (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 229:1822–1830. https://doi.org/10.1002/jcp.24636
Hernandez CJ, Guss JD, Luna M et al (2016) Links between the microbiome and bone. J Bone Miner Res 31:1638–1646. https://doi.org/10.1002/jbmr.2887
Govender M, Choonara YE, Kumar P et al (2014) A review of the advancements in probiotic delivery: conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech 15:29–43. https://doi.org/10.1208/s12249-013-0027-1
Chen YC, Greenbaum J, Shen H et al (2017) Association between gut microbiota and bone health: potential mechanisms and prospective. J Clin Endocrinol Metabol 102:3635–3646. https://doi.org/10.1210/jc.2017-00513
Parvaneh K, Jamaluddin R, Karimi G et al (2014) Effect of probiotics supplementation on bone mineral content and bone mass density. Sci World J 2014:595962. https://doi.org/10.1155/2014/595962
D’Amelio P, Sassi F (2018) Gut microbiota, immune system, and bone. Calcif Tissue Int 102:415–425. https://doi.org/10.1007/s00223-017-0331-y
Hsu E, Pacifici R (2018) From osteoimmunology to osteomicrobiology: how the microbiota and the immune system regulate bone. Calcif Tissue Int 102:512–521. https://doi.org/10.1007/s00223-017-0321-0
Collins FL, Rios-Arce ND, Schepper JD et al (2017) The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr 5:4. https://doi.org/10.1128/microbiolspec.BAD-0015-2016
Jafarnejad S, Djafarian K, Fazeli MR et al (2017) Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr 36:497–506. https://doi.org/10.1080/07315724.2017.1318724
Zhang J, Motyl KJ, Irwin R et al (2015) Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology 156:3169–3182. https://doi.org/10.1210/en.2015-1308
Ohlsson C, Engdahl C, Fak F et al (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 9:e92368. https://doi.org/10.1371/journal.pone.0092368
Zhang J, Lu Y, Wang Y et al (2018) The impact of the intestinal microbiome on bone health. Intractable Rare Dis Res 7:148–155. https://doi.org/10.5582/irdr.2018.01055
Kim JG, Lee E, Kim SH et al (2009) Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J 19:690–695
Parvaneh K, Ebrahimi M, Sabran MR et al (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:897639. https://doi.org/10.1155/2015/897639
Steves CJ, Bird S, Williams FM et al (2016) The microbiome and musculoskeletal conditions of aging: a review of evidence for impact and potential therapeutics. J Bone Miner Res 31:261–269. https://doi.org/10.1002/jbmr.2765
Sjogren K, Engdahl C, Henning P et al (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367. https://doi.org/10.1002/jbmr.1588
Aurigemma NC, Koltun KJ, VanEvery H et al (2018) Linking the gut microbiota to bone health in anorexia nervosa. Curr Osteoporos Rep 16:65–75. https://doi.org/10.1007/s11914-018-0420-5
Chiang SS, Pan TM (2011) Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem 59:7734–7742. https://doi.org/10.1021/jf2013716
Rizzoli R, Biver E (2018) Effects of fermented milk products on bone. Calcif Tissue Int 102:489–500. https://doi.org/10.1007/s00223-017-0317-9
McNulty NP, Yatsunenko T, Hsiao A et al (2011) The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med 3:106ra106. https://doi.org/10.1126/scitranslmed.3002701
Dar HY, Shukla P, Mishra PK et al (2018) Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep 8:46–56. https://doi.org/10.1016/j.bonr.2018.02.001
Collins FL, Irwin R, Bierhalter H et al (2016) Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS ONE 11:e0153180
Yousf H, Tomar G, Kr Srivastava R (2015) Probiotics and bone health: it takes GUTS to improve bone density. Int J Immunother Cancer Res 1:18–22
Yi J, Tang R, Yang J et al (2019) Streptolysin O derived from Streptococcus pyogenes inhibits RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Mol Med Rep 19:414–422
Schepper JD, Irwin R, Kang J et al (2017) Probiotics in gut-bone signaling. Adv Exp Med Biol 1033:225–247. https://doi.org/10.1007/978-3-319-66653-2_11
Liu TH, Tsai TY, Pan TM (2018) The anti-periodontitis effects of ethanol extract prepared using Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients. https://doi.org/10.3390/nu10040472
Shim KS, Kim T, Ha H et al (2012) Hwangryun–Haedok–Tang fermented with Lactobacillus casei suppresses ovariectomy-induced bone loss. Evid Based Complement Alternat Med eCAM 2012:325791. https://doi.org/10.1155/2012/325791
Wang S, Chin Heng B, Qiu S et al (2018) Lipoteichoic acid of Enterococcus faecalis inhibits osteoclastogenesis via transcription factor RBP-J. Innate Immun. https://doi.org/10.1177/1753425918812646
Foureaux Rde C, Messora MR, de Oliveira LF et al (2014) Effects of probiotic therapy on metabolic and inflammatory parameters of rats with ligature-induced periodontitis associated with restraint stress. J Periodontol 85:975–983. https://doi.org/10.1902/jop.2013.130356
Li JY, Chassaing B, Tyagi AM et al (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Investig 126:2049–2063. https://doi.org/10.1172/jci86062
Takimoto T, Hatanaka M, Hoshino T et al (2018) Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health 37:87–96
Acknowledgements
We are grateful to our colleagues for their patience and their advice on searching the papers.
Author information
Authors and Affiliations
Contributions
All authors contributed to all parts of the present review including papers selection, information synthesis, and paper drafting/editing. All authors approved and verified the final version.
Corresponding author
Ethics declarations
Conflict of interests
All authors declare that they have no conflict of interest.
Statement of human and animal rights
Not applicable.
Informed consent
All authors gave their informed consent prior to their inclusion in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amin, N., Boccardi, V., Taghizadeh, M. et al. Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res 32, 363–371 (2020). https://doi.org/10.1007/s40520-019-01223-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01223-5